Caveolin-1 gene therapy inhibits inflammasome activation to protect from bleomycin-induced pulmonary fibrosis
- PMID: 31873099
- PMCID: PMC6928213
- DOI: 10.1038/s41598-019-55819-y
Caveolin-1 gene therapy inhibits inflammasome activation to protect from bleomycin-induced pulmonary fibrosis
Abstract
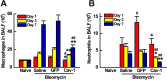
Idiopathic pulmonary fibrosis (IPF) is a devastating and fatal disease and characterized by increased deposition of extracellular matrix proteins and scar formation in the lung, resulting from alveolar epithelial damage and accumulation of inflammatory cells. Evidence suggests that Caveolin-1 (Cav-1), a major component of caveolae which regulates cell signaling and endocytosis, is a potential target to treat fibrotic diseases, although the mechanisms and responsible cell types are unclear. We show that Cav-1 expression was downregulated both in alveolar epithelial type I cells in bleomycin-injured mouse lungs and in lung sections from IPF patients. Increased expression of IL-1β and caspase-1 has been observed in IPF patients, indicating inflammasome activation associated with IPF. Gene transfer of a plasmid expressing Cav-1 using transthoracic electroporation reduced infiltration of neutrophils and monocytes/macrophages and protected from subsequent bleomycin-induced pulmonary fibrosis. Overexpression of Cav-1 suppressed bleomycin- or silica-induced activation of caspase-1 and maturation of pro-IL-1β to secrete cleaved IL-1β both in mouse lungs and in primary type I cells. These results demonstrate that gene transfer of Cav-1 downregulates inflammasome activity and protects from subsequent bleomycin-mediated pulmonary fibrosis. This indicates a pivotal regulation of Cav-1 in inflammasome activity and suggests a novel therapeutic strategy for patients with IPF.
Conflict of interest statement
Dr. Sime reports grants from NIH, during the conduct of the study; grants from NIH, grants and personal fees from UCB, personal fees from Boehringer Ingelheim, personal fees from Intermune/roche, personal fees from GSK, outside the submitted work. No other authors have any competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

