Structural basis of methotrexate and pemetrexed action on serine hydroxymethyltransferases revealed using plant models
- PMID: 31873125
- PMCID: PMC6928210
- DOI: 10.1038/s41598-019-56043-4
Structural basis of methotrexate and pemetrexed action on serine hydroxymethyltransferases revealed using plant models
Abstract
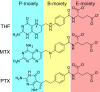
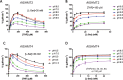
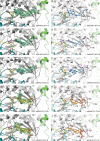
Serine hydroxymethyltransferases (SHMTs) reversibly transform serine into glycine in a reaction accompanied with conversion of tetrahydrofolate (THF) into 5,10-methylene-THF (5,10-meTHF). In vivo, 5,10-meTHF is the main carrier of one-carbon (1C) units, which are utilized for nucleotide biosynthesis and other processes crucial for every living cell, but hyperactivated in overproliferating cells (e.g. cancer tissues). SHMTs are emerging as a promising target for development of new drugs because it appears possible to inhibit growth of cancer cells by cutting off the supply of 5,10-meTHF. Methotrexate (MTX) and pemetrexed (PTX) are two examples of antifolates that have cured many patients over the years but target different enzymes from the folate cycle (mainly dihydrofolate reductase and thymidylate synthase, respectively). Here we show crystal structures of MTX and PTX bound to plant SHMT isozymes from cytosol and mitochondria-human isozymes exist in the same subcellular compartments. We verify inhibition of the studied isozymes by a thorough kinetic analysis. We propose to further exploit antifolate scaffold in development of SHMT inhibitors because it seems likely that especially polyglutamylated PTX inhibits SHMTs in vivo. Structure-based optimization is expected to yield novel antifolates that could potentially be used as chemotherapeutics.
Conflict of interest statement
The authors declare no competing interests.
Figures


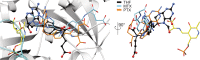

Similar articles
-
Serine hydroxymethyltransferase isoforms are differentially inhibited by leucovorin: characterization and comparison of recombinant zebrafish serine hydroxymethyltransferases.Drug Metab Dispos. 2007 Nov;35(11):2127-37. doi: 10.1124/dmd.107.016840. Epub 2007 Jul 30. Drug Metab Dispos. 2007. PMID: 17664250
-
In silico and in vitro validation of serine hydroxymethyltransferase as a chemotherapeutic target of the antifolate drug pemetrexed.Eur J Med Chem. 2011 May;46(5):1616-21. doi: 10.1016/j.ejmech.2011.02.009. Epub 2011 Mar 2. Eur J Med Chem. 2011. PMID: 21371789
-
Folate polyglutamylation eliminates dependence of activity on enzyme concentration in mitochondrial serine hydroxymethyltransferases from Arabidopsis thaliana.Arch Biochem Biophys. 2013 Aug 1;536(1):87-96. doi: 10.1016/j.abb.2013.06.004. Epub 2013 Jun 22. Arch Biochem Biophys. 2013. PMID: 23800877
-
Anticancer antifolates: current status and future directions.Curr Pharm Des. 2003;9(31):2593-613. doi: 10.2174/1381612033453712. Curr Pharm Des. 2003. PMID: 14529544 Review.
-
Deaza analogs of folic acid as antitumor agents.Curr Pharm Des. 2003;9(31):2615-25. doi: 10.2174/1381612033453695. Curr Pharm Des. 2003. PMID: 14529545 Review.
Cited by
-
Overcoming Radiation Resistance in Gliomas by Targeting Metabolism and DNA Repair Pathways.Int J Mol Sci. 2022 Feb 17;23(4):2246. doi: 10.3390/ijms23042246. Int J Mol Sci. 2022. PMID: 35216362 Free PMC article. Review.
-
Revealing protonation states and tracking substrate in serine hydroxymethyltransferase with room-temperature X-ray and neutron crystallography.Commun Chem. 2023 Aug 3;6(1):162. doi: 10.1038/s42004-023-00964-9. Commun Chem. 2023. PMID: 37532884 Free PMC article.
-
Insights into Metabolic Reprogramming in Tumor Evolution and Therapy.Cancers (Basel). 2024 Oct 17;16(20):3513. doi: 10.3390/cancers16203513. Cancers (Basel). 2024. PMID: 39456607 Free PMC article. Review.
-
Universality of critical active site glutamate as an acid-base catalyst in serine hydroxymethyltransferase function.Chem Sci. 2024 Jul 3;15(32):12827-12844. doi: 10.1039/d4sc03187c. eCollection 2024 Aug 14. Chem Sci. 2024. PMID: 39148791 Free PMC article.
-
Effect of Light Quality on Metabolomic, Ionomic, and Transcriptomic Profiles in Tomato Fruit.Int J Mol Sci. 2022 Oct 31;23(21):13288. doi: 10.3390/ijms232113288. Int J Mol Sci. 2022. PMID: 36362073 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

