Electron Tomography Analysis of Thylakoid Assembly and Fission in Chloroplasts of a Single-Cell C4 plant, Bienertia sinuspersici
- PMID: 31873131
- PMCID: PMC6927967
- DOI: 10.1038/s41598-019-56083-w
Electron Tomography Analysis of Thylakoid Assembly and Fission in Chloroplasts of a Single-Cell C4 plant, Bienertia sinuspersici
Abstract
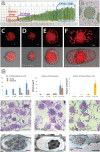
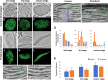
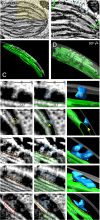
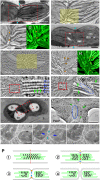
Bienertia sinuspersici is a single-cell C4 plant species of which chlorenchyma cells have two distinct groups of chloroplasts spatially segregated in the cytoplasm. The central vacuole encloses most chloroplasts at the cell center and confines the rest of the chloroplasts near the plasma membrane. Young chlorenchyma cells, however, do not have large vacuoles and their chloroplasts are homogenous. Therefore, maturing Bienertia chlorenchyma cells provide a unique opportunity to investigate chloroplast proliferation in the central cluster and the remodeling of chloroplasts that have been displaced by the vacuole to the cell periphery. Chloroplast numbers and sizes increased, more notably, during later stages of maturation than the early stages. Electron tomography analyses indicated that chloroplast enlargement is sustained by thylakoid growth and that invaginations from the inner envelope membrane contributed to thylakoid assembly. Grana stacks acquired more layers, differentiating them from stroma thylakoids as central chloroplasts matured. In peripheral chloroplasts, however, grana stacks stretched out to a degree that the distinction between grana stacks and stroma thylakoids was obscured. In central chloroplasts undergoing division, thylakoids inside the cleavage furrow were kinked and severed. Grana stacks in the division zone were disrupted, and large complexes in their membranes were dislocated, suggesting the existence of a thylakoid fission machinery.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Voznesenskaya EV, Franceschi VR, Pyankov VI, Edwards GE. Anatomy, chloroplast structure and compartmentation of enzymes relative to photosynthetic mechanisms in leaves and cotyledons of species in the tribe Salsoleae (Chenopodiaceae) J Exp Bot. 1999;50:1779–1795. doi: 10.1093/jxb/50.341.1779. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

