Functional impact of cytochrome P450 3A (CYP3A) missense variants in cattle
- PMID: 31873175
- PMCID: PMC6927969
- DOI: 10.1038/s41598-019-56271-8
Functional impact of cytochrome P450 3A (CYP3A) missense variants in cattle
Abstract
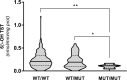
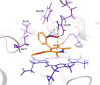
Cytochrome P450 3A is the most important CYP subfamily in humans, and CYP3A4/CYP3A5 genetic variants contribute to inter-individual variability in drug metabolism. However, no information is available for bovine CYP3A (bCYP3A). Here we described bCYP3A missense single nucleotide variants (SNVs) and evaluated their functional effects. CYP3A28, CYP3A38 and CYP3A48 missense SNVs were identified in 300 bulls of Piedmontese breed through targeted sequencing. Wild-type and mutant bCYP3A cDNAs were cloned and expressed in V79 cells. CYP3A-dependent oxidative metabolism of testosterone (TST) and nifedipine (NIF) was assessed by LC-MS/MS. Finally, SNVs functional impact on TST hydroxylation was measured ex vivo in liver microsomes from individually genotyped animals. Thirteen missense SNVs were identified and validated. Five variants showed differences in CYP3A catalytic activity: three CYP3A28 SNVs reduced TST 6β-hydroxylation; one CYP3A38 variant increased TST 16β-hydroxylation, while a CYP3A48 SNV showed enhanced NIF oxidation. Individuals homozygous for rs384467435 SNV showed a reduced TST 6β-hydroxylation. Molecular modelling showed that most of SNVs were distal to CYP3A active site, suggesting indirect effects on the catalytic activity. Collectively, these findings demonstrate the importance of pharmacogenetics studies in veterinary species and suggest bCYP3A genotype variation might affect the fate of xenobiotics in food-producing species such as cattle.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Impact of Missense Mutations on AFB1 Metabolism in Bovine Cytochrome P4503A Isoforms: A Computational Mutagenesis and Molecular Docking Analysis.Int J Mol Sci. 2024 Nov 22;25(23):12529. doi: 10.3390/ijms252312529. Int J Mol Sci. 2024. PMID: 39684241 Free PMC article.
-
Defective activity of recombinant cytochromes P450 3A4.2 and 3A4.16 in oxidation of midazolam, nifedipine, and testosterone.Drug Metab Dispos. 2008 Nov;36(11):2287-91. doi: 10.1124/dmd.108.021816. Epub 2008 Jul 31. Drug Metab Dispos. 2008. PMID: 18669585
-
Comparison of steroid hormone hydroxylation mediated by cytochrome P450 3A subfamilies.Arch Biochem Biophys. 2020 Mar 30;682:108283. doi: 10.1016/j.abb.2020.108283. Epub 2020 Jan 27. Arch Biochem Biophys. 2020. PMID: 32001245
-
Functional characterization of medaka CYP3A38 and CYP3A40: kinetics and catalysis by expression in a recombinant baculovirus system.Comp Biochem Physiol C Toxicol Pharmacol. 2005 Aug;141(4):338-48. doi: 10.1016/j.cca.2005.07.006. Epub 2005 Aug 22. Comp Biochem Physiol C Toxicol Pharmacol. 2005. PMID: 16112913
-
Why We Need to Take a Closer Look at Genetic Contributions to CYP3A Activity.Front Pharmacol. 2022 Jun 16;13:912618. doi: 10.3389/fphar.2022.912618. eCollection 2022. Front Pharmacol. 2022. PMID: 35784699 Free PMC article. Review.
Cited by
-
Hypermethylation-Mediated Silencing of CIDEA, MAL and PCDH17 Tumour Suppressor Genes in Canine DLBCL: From Multi-Omics Analyses to Mechanistic Studies.Int J Mol Sci. 2022 Apr 5;23(7):4021. doi: 10.3390/ijms23074021. Int J Mol Sci. 2022. PMID: 35409379 Free PMC article.
-
Impact of Missense Mutations on AFB1 Metabolism in Bovine Cytochrome P4503A Isoforms: A Computational Mutagenesis and Molecular Docking Analysis.Int J Mol Sci. 2024 Nov 22;25(23):12529. doi: 10.3390/ijms252312529. Int J Mol Sci. 2024. PMID: 39684241 Free PMC article.
-
Ethnic Aspects of Valproic Acid P-Oxidation.Biomedicines. 2024 May 8;12(5):1036. doi: 10.3390/biomedicines12051036. Biomedicines. 2024. PMID: 38790997 Free PMC article. Review.
-
Single Nucleotide Polymorphism Induces Divergent Dynamic Patterns in CYP3A5: A Microsecond Scale Biomolecular Simulation of Variants Identified in Sub-Saharan African Populations.Int J Mol Sci. 2021 Jul 21;22(15):7786. doi: 10.3390/ijms22157786. Int J Mol Sci. 2021. PMID: 34360551 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

