Impact of Surgical Margins in Breast Cancer After Preoperative Systemic Chemotherapy on Local Recurrence and Survival
- PMID: 31873929
- PMCID: PMC7138765
- DOI: 10.1245/s10434-019-08089-x
Impact of Surgical Margins in Breast Cancer After Preoperative Systemic Chemotherapy on Local Recurrence and Survival
Abstract
Background: While "no tumour on ink" is an accepted margin width for R0 resection in primary surgery, it's unclear if it's oncologically safe after neoadjuvant chemotherapy (NAC). Only limited data demonstrate that surgery within new margins in cases of a pathological complete response (pCR) is safe. We therefore investigated the influence of different margins and pCR on local recurrence and survival rates after NAC.
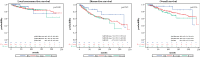
Methods: We retrospectively analysed data of 406 women with invasive breast cancer, treated with NAC and breast-conserving therapy between 1994 and 2014 in two certified Austrian breast health centres. We compared R ≤ 1 mm, R > 1 mm and RX (pCR) for local recurrence-free survival (LRFS), disease-free survival (DFS) and overall survival (OS).
Results: After a median follow-up of 84.3 months, the 5-year LRFS (R ≤ 1 mm: 94.2%, R > 1 mm: 90.6%, RX: 95.0%; p = 0.940), the 5-year DFS (R ≤ 1 mm: 71.9%, R > 1 mm: 74.1%, RX: 87.2%; p = 0.245) and the 5-year OS (R ≤ 1 mm: 85.1%, R > 1 mm: 88.0%, RX: 96.4%; p = 0.236) did not differ significantly between narrow, wide, nor RX resections. Regarding DFS and OS, a negative nodal status reduced the hazard ratio significantly.
Conclusion: There is no significant difference in LRFS, DFS and OS comparing close, wide or unknown margins after pCR. We suggest that resection in new margins after NAC is safe according to "no tumour on ink". Resection of the clipped area in cases of pCR is emphasized.
Conflict of interest statement
Dr. Wimmer reports travel grants from Roche and Pfizer, outside the submitted work. Dr. Bolliger reports personal fees from Pfizer for congress support, outside the submitted work. Dr. Bago-Horvath reports other from Roche, grants from Boehringer-Ingelheim and personal fees from Novartis, outside the submitted work. Dr. Kauer-Dorner reports personal fees from Roche, outside the submitted work. Dr. Fitzal reports support from Roche, from Pfizer, from Novartis, from Astra Zeneca and from Polytech for advisery boards, meetings, and lectures, outside the submitted work. Dr. Steger, Dr. Helfgott, Dr. Gruber, Dr. Moinfar and Dr. Mittlböck have nothing to disclose. Regarding this work, none of the authors reports any conflicts of interest.
Figures


References
-
- Gralow JR, Burstein HJ, Wood W, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2008;26(5):814–819. doi: 10.1200/JCO.2007.15.3510. - DOI - PubMed
-
- Kaufmann M, Morrow M, von Minckwitz G, Harris JR, Biedenkopf Expert Panel M. Locoregional treatment of primary breast cancer: consensus recommendations from an international expert panel. Cancer. 2010;116(5):1184–91. - PubMed
-
- Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology—American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2014;32(14):1507–1515. doi: 10.1200/JCO.2013.53.3935. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

