Functionalization of Piperidine Derivatives for the Site-Selective and Stereoselective Synthesis of Positional Analogues of Methylphenidate
- PMID: 31873946
- PMCID: PMC7187323
- DOI: 10.1002/chem.201905773
Functionalization of Piperidine Derivatives for the Site-Selective and Stereoselective Synthesis of Positional Analogues of Methylphenidate
Abstract
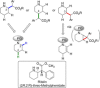
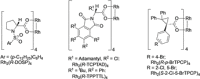
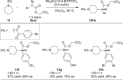
Rhodium-catalyzed C-H insertions and cyclopropanations of donor/acceptor carbenes have been used for the synthesis of positional analogues of methylphenidate. The site selectivity is controlled by the catalyst and the amine protecting group. C-H functionalization of N-Boc-piperidine using Rh2 (R-TCPTAD)4 , or N-brosyl-piperidine using Rh2 (R-TPPTTL)4 generated 2-substitited analogues. In contrast, when N-α-oxoarylacetyl-piperidines were used in combination with Rh2 (S-2-Cl-5-BrTPCP)4 , the C-H functionalization produced 4-susbstiuted analogues. Finally, the 3-substituted analogues were prepared indirectly by cyclopropanation of N-Boc-tetrahydropyridine followed by reductive regio- and stereoselective ring-opening of the cyclopropanes.
Keywords: C−H functionalization; diastereoselectivity; piperidines; regioselectivity; rhodium.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
HMLD is a named inventor on a patent entitled, Dirhodium Catalyst Compositions and Synthetic Processes Related Thereto (US 8,974,428, issued 3/10/2015). The other authors have no competing financial interests.
Figures

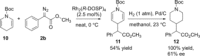
References
Grants and funding
LinkOut - more resources
Full Text Sources

