Substrates, inhibitors, and probes of mammalian transglutaminase 2
- PMID: 31874171
- PMCID: PMC6948143
- DOI: 10.1016/j.ab.2019.113560
Substrates, inhibitors, and probes of mammalian transglutaminase 2
Abstract
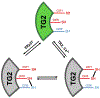
Transglutaminase 2 (TG2) is a ubiquitous but enigmatic mammalian protein to which a number of biological functions have been ascribed but not definitively proven. As a member of the transglutaminase family, TG2 can catalyze deamidation or alternatively transamidation of selected Gln residues in proteins and peptides. It is also known to harbor other enzymatic properties, including protein disulfide isomerase, GTP-dependent signal transduction, and ATP dependent protein kinase activity. Given its multifunctional chemistry, it is unsurprising that a long list of proteins from the mammalian proteome have been identified as substrates and/or binding partners; however, the biological relevance of none of these protein-protein interactions has been clarified as yet. Remarkably, the most definitive insights into the biology of TG2 stem from its pathophysiological role in gluten peptide deamidation in celiac disease. Meanwhile our understanding of TG2 chemistry has been leveraged to engineer a spectrum of inhibitors and other molecular probes of TG2 biology in vivo. This review summarizes our current knowledge of the enzymology and regulation of human TG2 with a focus on its physiological substrates as well as tool molecules whose engineering was inspired by their identities.
Keywords: Chemical biology; Post-translational modifications; Transglutaminase.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures

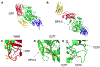
References
-
- Achyuthan KE, Greenberg CS, Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity, J Biol Chem 262 (1987) 1901–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

