Clinical and economic outcomes among injection-naïve patients with type 2 diabetes initiating dulaglutide compared with basal insulin in a US real-world setting: the DISPEL Study
- PMID: 31875137
- PMCID: PMC6904197
- DOI: 10.1136/bmjdrc-2019-000884
Clinical and economic outcomes among injection-naïve patients with type 2 diabetes initiating dulaglutide compared with basal insulin in a US real-world setting: the DISPEL Study
Abstract
Aims: To report 1-year clinical and economic outcomes from the retrospective DISPEL (Dulaglutide vs Basal InSulin in Injection Naïve Patients with Type 2 Diabetes: Effectiveness in ReaL World) Study.
Materials and methods: This observational claims study included patients with type 2 diabetes (T2D) and ≥1 claim for dulaglutide or basal insulin between November 2014 and April 2017 (index date=earliest fill date). Propensity score matching was used to address treatment selection bias. Change from baseline in hemoglobin A1c (HbA1c) was compared between the matched cohorts using analysis of covariance; diabetes-related costs were analyzed using generalized linear models.
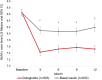
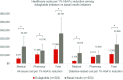
Results: Matched cohorts (903 pairs total; 523 pairs with complete cost data) were balanced in baseline characteristics with mean HbA1c 8.6%, mean age 54 years. At 1 year postindex, dulaglutide patients had significantly greater reduction in HbA1c than basal insulin (-1.12% vs -0.51%, p<0.01), lower medical costs ($3753 vs $7604, p<0.01), higher pharmacy costs ($9809 vs $6175, p<0.01), and similar total costs ($13 562 vs $13 779, p=0.76). Medical and total costs per 1% HbA1c reduction were lower for dulaglutide than basal insulin (medical: $3128 vs $12 673, p<0.01; total: $11 302 vs $22 965, p<0.01), while pharmacy costs per 1% HbA1c reduction were lower without reaching statistical significance ($8174 vs $10 292, p=0.15).
Conclusions: In this real-world study, patients with T2D initiating dulaglutide demonstrated greater HbA1c reduction compared with those initiating basal insulin. Although total diabetes-related costs were similar, the total diabetes-related costs per HbA1c reduction were lower for dulaglutide, highlighting the importance of evaluating effectiveness along with the economic impact of medications.
Keywords: HbA1c; adherence to medications; claims database analysis; insulin delivery.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RM, MY and HP are employees and stockholders of Eli Lilly and Company. MG is an employee of HealthCore, an independent research organization that received funding from Eli Lilly and Company for the conduct of this study. QH, XZ and LW were employees of HealthCore at the time the study was conducted.
Figures

References
-
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. . Consensus statement by the american association of clinical endocrinologists and american college of endocrinology on the comprehensive type 2 diabetes management algorithm - 2019 EXECUTIVE SUMMARY. Endocr Pract 2019;25:69–100. 10.4158/CS-2018-0535 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
