Electrophotocatalytic Undirected C-H Trifluoromethylations of (Het)Arenes
- PMID: 31875327
- PMCID: PMC7155051
- DOI: 10.1002/chem.201905774
Electrophotocatalytic Undirected C-H Trifluoromethylations of (Het)Arenes
Abstract
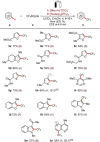
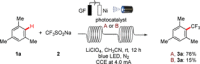
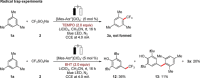
Electrophotochemistry has enabled arene C-H trifluoromethylation with the Langlois reagent CF3 SO2 Na under mild reaction conditions. The merger of electrosynthesis and photoredox catalysis provided a chemical oxidant-free approach for the generation of the CF3 radical. The electrophotochemistry was carried out in an operationally simple manner, setting the stage for challenging C-H trifluoromethylations of unactivated arenes and heteroarenes. The robust nature of the electrophotochemical manifold was reflected by a wide scope, including electron-rich and electron-deficient benzenes, as well as naturally occurring heteroarenes. Electrophotochemical C-H trifluoromethylation was further achieved in flow with a modular electro-flow-cell equipped with an in-operando monitoring unit for on-line flow-NMR spectroscopy, providing support for the single electron transfer processes.
Keywords: C−H trifluoromethylation; arenes; catalysis; electrophotochemistry; oxidant-free.
© 2019 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Direct Arene Trifluoromethylation Enabled by a High-Valent CuIII -CF3 Compound.Angew Chem Int Ed Engl. 2022 Oct 4;61(40):e202209029. doi: 10.1002/anie.202209029. Epub 2022 Aug 29. Angew Chem Int Ed Engl. 2022. PMID: 35939056
-
Trifluoromethylations of (Hetero)arenes and Polarized Alkenes Using Trifluoroacetic Anhydride under Photoredox Catalysis.Org Lett. 2023 Apr 7;25(13):2372-2376. doi: 10.1021/acs.orglett.3c00890. Epub 2023 Mar 27. Org Lett. 2023. PMID: 36971301
-
Catalytic C-H Trifluoromethylation of Arenes and Heteroarenes via Visible Light Photoexcitation of a Co(III)-CF3 Complex.ACS Catal. 2023 Oct 9;13(20):13607-13617. doi: 10.1021/acscatal.3c03832. eCollection 2023 Oct 20. ACS Catal. 2023. PMID: 37881792 Free PMC article.
-
Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl)trimethylsilane as a nucleophilic CF3 source.Acc Chem Res. 2014 May 20;47(5):1513-22. doi: 10.1021/ar4003202. Epub 2014 Apr 28. Acc Chem Res. 2014. PMID: 24773518 Review.
-
Functionalization of C-H Bonds by Photoredox Catalysis.Chem Rec. 2017 Aug;17(8):754-774. doi: 10.1002/tcr.201600125. Epub 2017 Jan 11. Chem Rec. 2017. PMID: 28074599 Review.
Cited by
-
Photoelectrochemical oxidative C(sp3)-H borylation of unactivated hydrocarbons.Nat Commun. 2023 Oct 16;14(1):6530. doi: 10.1038/s41467-023-42264-9. Nat Commun. 2023. PMID: 37845202 Free PMC article.
-
Synthetic Molecular Photoelectrochemistry: New Frontiers in Synthetic Applications, Mechanistic Insights and Scalability.Angew Chem Int Ed Engl. 2022 Mar 14;61(12):e202107811. doi: 10.1002/anie.202107811. Epub 2022 Feb 9. Angew Chem Int Ed Engl. 2022. PMID: 34478188 Free PMC article. Review.
-
Recent Advances in Rapid Synthesis of Non-proteinogenic Amino Acids from Proteinogenic Amino Acids Derivatives via Direct Photo-Mediated C-H Functionalization.Molecules. 2020 Nov 12;25(22):5270. doi: 10.3390/molecules25225270. Molecules. 2020. PMID: 33198166 Free PMC article. Review.
-
Electrophotocatalytic perfluoroalkylation by LMCT excitation of Ag(II) perfluoroalkyl carboxylates.Science. 2024 Jan 19;383(6680):279-284. doi: 10.1126/science.adk4919. Epub 2023 Dec 14. Science. 2024. PMID: 38096334 Free PMC article.
-
Renewable resources for sustainable metallaelectro-catalysed C-H activation.Chem Sci. 2020 Jul 31;11(33):8657-8670. doi: 10.1039/d0sc03578e. Chem Sci. 2020. PMID: 34123124 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

