Meta-analysis and review of functional neuroimaging differences underlying adolescent vulnerability to substance use
- PMID: 31875520
- PMCID: PMC7992390
- DOI: 10.1016/j.neuroimage.2019.116476
Meta-analysis and review of functional neuroimaging differences underlying adolescent vulnerability to substance use
Abstract
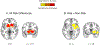
Adolescence is increasingly viewed as a sensitive period in the development of substance use disorders (SUDs). Neurodevelopmental 'dual-risk' theories suggest adolescent vulnerability to problematic substance use is driven by an overactive reward drive mediated by the striatum, and poor cognitive control mediated by the prefrontal cortex. To this end, there has been a growing number of neuroimaging studies examining cognitive and affective neural systems during adolescence for markers of vulnerability to problematic substance use. Here, we perform a coordinate-based meta-analysis on this emerging literature. Twenty-two task-based voxelwise fMRI studies with activation differences associated with substance use vulnerability, representative of approximately 1092 subjects, were identified through a systematic literature search (PubMed, Scopus) and coordinates of activation differences (N = 190) were extracted. Adolescents were defined as 'at-risk' for problematic substance use based on a family history of SUD or through prospective prediction of substance use initiation or escalation. Multilevel kernel density analysis was used to identify the most consistent brain regions associated with adolescent substance use vulnerability. Across the included studies, substance use vulnerability was most reliably associated with activation differences in the striatum, where at-risk adolescents had hyper-activation in the dorsal subdivision (putamen). Follow-up analyses suggested striatal differences were driven by tasks sharing a motivational and/or reward component (e.g., monetary incentive) and common across subgroups of substance use risk (family history and prospective prediction studies). Analyses examining the role of psychiatric comorbidity revealed striatal activation differences were significantly more common in samples whose definition of substance use risk included cooccurring externalizing psychopathology. Furthermore, substance use risk meta-analytic results were no longer significant when excluding these studies, although this may reflect limitations in statistical power. No significant activation differences were observed in prefrontal cortex in any analysis. These results suggest striatal dysfunction, rather than prefrontal, may be a more primary neural feature of adolescent vulnerability to problematic substance use, possibly through a dimension of individual variability shared with externalizing psychopathology. However, our systematic literature search confirms this is still an emerging field. More studies, increased data sharing, and further quantitative integration are necessary for a comprehensive understanding of the neuroimaging markers of adolescent substance use risk.
Keywords: Addiction; Adolescence; Children of alcoholics; Cognitive control; Reward; Striatum; Substance use disorder; Substance use risk; fMRI; meta-Analysis.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors report no financial relationships with commercial interests.
Figures
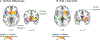


Similar articles
-
Neural systems underlying reward cue processing in early adolescence: The role of puberty and pubertal hormones.Psychoneuroendocrinology. 2019 Apr;102:281-291. doi: 10.1016/j.psyneuen.2018.12.016. Epub 2018 Dec 13. Psychoneuroendocrinology. 2019. PMID: 30639923 Free PMC article.
-
Structural and Functional Neural Targets of Addiction Treatment in Adolescents and Young Adults: A Systematic Review and Meta-Analysis.J Child Adolesc Psychopharmacol. 2019 Aug;29(7):498-507. doi: 10.1089/cap.2019.0007. Epub 2019 Jul 16. J Child Adolesc Psychopharmacol. 2019. PMID: 31313938 Free PMC article.
-
Common and separable neural alterations in substance use disorders: A coordinate-based meta-analyses of functional neuroimaging studies in humans.Hum Brain Mapp. 2020 Nov;41(16):4459-4477. doi: 10.1002/hbm.25085. Epub 2020 Sep 10. Hum Brain Mapp. 2020. PMID: 32964613 Free PMC article.
-
Common neurofunctional dysregulations characterize obsessive-compulsive, substance use, and gaming disorders-An activation likelihood meta-analysis of functional imaging studies.Addict Biol. 2021 Jul;26(4):e12997. doi: 10.1111/adb.12997. Epub 2021 Jan 11. Addict Biol. 2021. PMID: 33432718
-
Striatocortical pathway dysfunction in addiction and obesity: differences and similarities.Crit Rev Biochem Mol Biol. 2013 Jan-Feb;48(1):1-19. doi: 10.3109/10409238.2012.735642. Epub 2012 Nov 23. Crit Rev Biochem Mol Biol. 2013. PMID: 23173916 Free PMC article. Review.
Cited by
-
Dopamine-related striatal neurophysiology is associated with specialization of frontostriatal reward circuitry through adolescence.Prog Neurobiol. 2021 Jun;201:101997. doi: 10.1016/j.pneurobio.2021.101997. Epub 2021 Mar 2. Prog Neurobiol. 2021. PMID: 33667595 Free PMC article.
-
Effects of family history of substance use disorder on reward processing in adolescents with and without attention-deficit/hyperactivity disorder.Addict Biol. 2022 Mar;27(2):e13137. doi: 10.1111/adb.13137. Addict Biol. 2022. PMID: 35229951 Free PMC article.
-
Behavioral and brain signatures of substance use vulnerability in childhood.Dev Cogn Neurosci. 2020 Dec;46:100878. doi: 10.1016/j.dcn.2020.100878. Epub 2020 Nov 3. Dev Cogn Neurosci. 2020. PMID: 33181393 Free PMC article.
-
Psychiatric neuroimaging designs for individualised, cohort, and population studies.Neuropsychopharmacology. 2024 Nov;50(1):29-36. doi: 10.1038/s41386-024-01918-y. Epub 2024 Aug 14. Neuropsychopharmacology. 2024. PMID: 39143320 Free PMC article. Review.
-
Developmental changes in dopamine-related neurophysiology and associations with adolescent substance use and incentive-boosted cognitive control.Dev Cogn Neurosci. 2025 Jul 4;75:101594. doi: 10.1016/j.dcn.2025.101594. Online ahead of print. Dev Cogn Neurosci. 2025. PMID: 40633164 Free PMC article.
References
-
- Alegria AA, Radua J, Rubia K, 2016. Meta-analysis of fMRI studies of disruptive behavior disorders. Am. J. Psychiatry 173, 1119–1130. - PubMed
-
- Andrews MM, Meda SA, Thomas AD, Potenza MN, Krystal JH, Worhunsky P, Stevens MC, O’Malley S, Book GA, Reynolds B, 2011. Individuals family history positive for alcoholism show functional magnetic resonance imaging differences in reward sensitivity that are related to impulsivity factors. Biol. Psychiatry 69, 675–683. - PMC - PubMed

