Axonal Odorant Receptors Mediate Axon Targeting
- PMID: 31875544
- PMCID: PMC6941231
- DOI: 10.1016/j.celrep.2019.11.099
Axonal Odorant Receptors Mediate Axon Targeting
Abstract
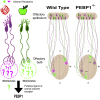
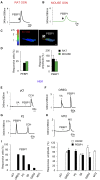
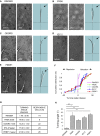
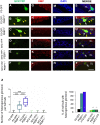
In mammals, odorant receptors not only detect odors but also define the target in the olfactory bulb, where sensory neurons project to give rise to the sensory map. The odorant receptor is expressed at the cilia, where it binds odorants, and at the axon terminal. The mechanism of activation and function of the odorant receptor at the axon terminal is, however, still unknown. Here, we identify phosphatidylethanolamine-binding protein 1 as a putative ligand that activates the odorant receptor at the axon terminal and affects the turning behavior of sensory axons. Genetic ablation of phosphatidylethanolamine-binding protein 1 in mice results in a strongly disturbed olfactory sensory map. Our data suggest that the odorant receptor at the axon terminal of olfactory neurons acts as an axon guidance cue that responds to molecules originating in the olfactory bulb. The dual function of the odorant receptor links specificity of odor perception and axon targeting.
Keywords: axon targeting; axonal odorant receptors; olfactory bulb; topographic map.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Al-Mulla F., Bitar M.S., Taqi Z., Yeung K.C. RKIP: much more than Raf kinase inhibitory protein. J. Cell. Physiol. 2013;228:1688–1702. - PubMed
-
- Barnea G., O’Donnell S., Mancia F., Sun X., Nemes A., Mendelsohn M., Axel R. Odorant receptors on axon termini in the brain. Science. 2004;304:1468. - PubMed
-
- Belluscio L., Gold G.H., Nemes A., Axel R. Mice deficient in G(olf) are anosmic. Neuron. 1998;20:69–81. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

