The relationship between miRNA-26b and connective tissue growth factor in rat models of aortic banding and debanding
- PMID: 31875666
- PMCID: PMC8137408
- DOI: 10.3904/kjim.2019.120
The relationship between miRNA-26b and connective tissue growth factor in rat models of aortic banding and debanding
Abstract
Background/aims: Connective tissue growth factor (CTGF) is a profibrotic factor implicated in pressure overload-mediated myocardial fibrosis. In this study, we determined the role of predicted CTGF-targeting microRNAs (miRNAs) in rat models of aortic stenosis and reverse cardiac remodeling.
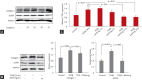
Methods: Minimally invasive ascending aortic banding was performed in 24 7-week-old male Sprague-Dawley rats, which were divided into three groups. The banding group consisted of eight rats that were sacrificed immediately after 6 weeks of aortic constriction. The debanding group underwent aortic constriction for 4 weeks and was sacrificed 2 weeks after band removal. The third group underwent sham surgery. We investigated the expression of CTGF, transforming growth factor-β1 (TGFβ1), and matrix metalloproteinase-2 using ELISA and examined miRNA-26b, miRNA-133a, and miRNA-19b as predicted CTGF-targeting miRNAs based on miRNA databases in 24-hour TGFβ-stimulated and TGFβ- washed fibroblasts and myocardial tissues from all subjects.
Results: CTGF was elevated in 24-hour TGFβ-stimulated fibroblasts and decreased in 24-hour TGFβ-washed fibroblasts. miRNA-26b was significantly increased in TGFβ-washed fibroblasts compared with control and TGFβ-stimulated fibroblasts (p < 0.05). CTGF expression was significantly higher in the banding group than that in the sham and debanding groups. The relative expression levels of miRNA-26b were higher in the debanding group than in the banding group.
Conclusion: The results of our study using models of aortic banding and debanding suggested that miRNA-26b was significantly increased after aortic debanding. The in vitro model yielded the same results: miRNA-26b was upregulated after removal of TGFβ from fibroblasts.
Keywords: Aortic banding; Aortic debanding; Connective tissue growth factor; Fibrosis; miRNA-26b.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures




Similar articles
-
Mitochondrial Reversible Changes Determine Diastolic Function Adaptations During Myocardial (Reverse) Remodeling.Circ Heart Fail. 2020 Nov;13(11):e006170. doi: 10.1161/CIRCHEARTFAILURE.119.006170. Epub 2020 Nov 12. Circ Heart Fail. 2020. PMID: 33176457
-
miR-19a, -19b, and -26b Mediate CTGF Expression and Pulmonary Fibroblast Differentiation.J Cell Physiol. 2016 Oct;231(10):2236-48. doi: 10.1002/jcp.25341. Epub 2016 Mar 14. J Cell Physiol. 2016. PMID: 26873752
-
miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling.Circ Res. 2009 Jan 30;104(2):170-8, 6p following 178. doi: 10.1161/CIRCRESAHA.108.182535. Epub 2008 Dec 18. Circ Res. 2009. PMID: 19096030
-
Ascending aortic adventitial remodeling and fibrosis are ameliorated with Apelin-13 in rats after TAC via suppression of the miRNA-122 and LGR4-β-catenin signaling.Peptides. 2016 Dec;86:85-94. doi: 10.1016/j.peptides.2016.10.005. Epub 2016 Oct 20. Peptides. 2016. PMID: 27773659
-
Transforming growth factor-beta/connective tissue growth factor axis in the kidney.Int J Biochem Cell Biol. 2008;40(1):9-13. doi: 10.1016/j.biocel.2007.01.006. Epub 2007 Jan 20. Int J Biochem Cell Biol. 2008. PMID: 17300978 Review.
Cited by
-
Rodent Models of Dilated Cardiomyopathy and Heart Failure for Translational Investigations and Therapeutic Discovery.Int J Mol Sci. 2023 Feb 5;24(4):3162. doi: 10.3390/ijms24043162. Int J Mol Sci. 2023. PMID: 36834573 Free PMC article. Review.
-
Clinical value of miR-216a-5p and miR-34a in early screening for cervical cancer.Am J Transl Res. 2025 Jan 15;17(1):462-470. doi: 10.62347/BCUC5946. eCollection 2025. Am J Transl Res. 2025. PMID: 39959236 Free PMC article.
References
-
- Houser SR, Margulies KB, Murphy AM, et al. Animal models of heart failure: a scientific statement from the American Heart Association. Circ Res. 2012;111:131–150. - PubMed
-
- Bjornstad JL, Skrbic B, Sjaastad I, Bjornstad S, Christensen G, Tonnessen T. A mouse model of reverse cardiac remodelling following banding-debanding of the ascending aorta. Acta Physiol (Oxf ) 2012;205:92–102. - PubMed
-
- Gao XM, Kiriazis H, Moore XL, et al. Regression of pressure overload-induced left ventricular hypertrophy in mice. Am J Physiol Heart Circ Physiol. 2005;288:H2702–H2707. - PubMed
-
- Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
