Rats genetically selected for low and high aerobic capacity exhibit altered soleus muscle myofilament functions
- PMID: 31875694
- PMCID: PMC7052611
- DOI: 10.1152/ajpcell.00430.2019
Rats genetically selected for low and high aerobic capacity exhibit altered soleus muscle myofilament functions
Abstract
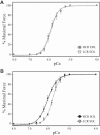
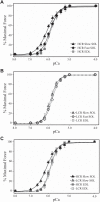
Aerobic exercise capacity is critical to bodily health. As a model to investigate the mechanisms that determine health and disease, we employed low (LCR) and high (HCR) capacity running rat models selectively bred to concentrate the genes responsible for divergent aerobic running capacity. To investigate the skeletal muscle contribution to this innate difference in running capacity we employed an approach combining examination of the myofilament protein composition and contractile properties of the fast fiber extensor digitorum longus (EDL) and slow fiber soleus (SOL) muscles from LCR and HCR rats. Intact muscle force experiments demonstrate that SOL, but not EDL, muscles from LCR rats exhibit a three times greater decrease in fatigued force. To investigate the mechanism of this increased fatigability in the LCR SOL muscle, we determined the myofilament protein composition and functional properties. Force-Ca2+ measurements demonstrate decreased Ca2+ sensitivity of single skinned SOL muscle fibers from LCR compared with that of HCR rats. Segregating SOL fibers into fast and slow types demonstrates that the decreased Ca2+ sensitivity in LCR SOL results from a specific decrease in slow-type SOL fiber Ca2+ sensitivity such that it was similar to that of fast-type fibers. These results identify that the altered myofilament contractile properties of LCR SOL slow-type fibers result in a fast muscle type Ca2+ sensitivity and the LCR muscle phenotype. Overall our findings demonstrate alterations of the myofilament proteins could contribute to fatigability of the SOL muscle and the decreased innate aerobic running performance of LCR compared with HCR rats.
Keywords: contractile proteins; muscle fatigue; myosin isoforms; single fiber; troponin isoforms.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Ackermann MA, Kerr JP, King B, Ward CW, Kontrogianni-Konstantopoulos A. The phosphorylation profile of myosin binding protein-C slow is dynamically regulated in slow-twitch muscles in health and disease. Sci Rep 5: 12637, 2015. [Erratum in Sci Rep 8: 46969, 2018]. doi:10.1038/srep12637. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

