A sensory appendage protein protects malaria vectors from pyrethroids
- PMID: 31875852
- PMCID: PMC6974402
- DOI: 10.1038/s41586-019-1864-1
A sensory appendage protein protects malaria vectors from pyrethroids
Abstract
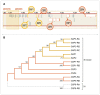
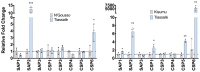
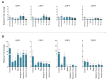
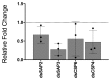
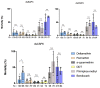
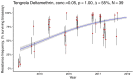
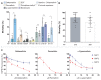
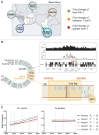
Pyrethroid-impregnated bed nets have driven considerable reductions in malaria-associated morbidity and mortality in Africa since the beginning of the century1. The intense selection pressure exerted by bed nets has precipitated widespread and escalating resistance to pyrethroids in African Anopheles populations, threatening to reverse the gains that been made by malaria control2. Here we show that expression of a sensory appendage protein (SAP2), which is enriched in the legs, confers pyrethroid resistance to Anopheles gambiae. Expression of SAP2 is increased in insecticide-resistant populations and is further induced after the mosquito comes into contact with pyrethroids. SAP2 silencing fully restores mortality of the mosquitoes, whereas SAP2 overexpression results in increased resistance, probably owing to high-affinity binding of SAP2 to pyrethroid insecticides. Mining of genome sequence data reveals a selective sweep near the SAP2 locus in the mosquito populations of three West African countries (Cameroon, Guinea and Burkina Faso) with the observed increase in haplotype-associated single-nucleotide polymorphisms mirroring the increasing resistance of mosquitoes to pyrethroids reported in Burkina Faso. Our study identifies a previously undescribed mechanism of insecticide resistance that is likely to be highly relevant to malaria control efforts.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Comment in
-
Malaria-carrying mosquitoes get a leg up on insecticides.Nature. 2020 Jan;577(7790):319-320. doi: 10.1038/d41586-019-03728-5. Nature. 2020. PMID: 31937951 No abstract available.
References
-
- WHO. World Malaria Report. Geneva: 2018.
-
- Hawley WA, et al. Community-wide effects of permethrin-treated bed nets on child mortality and malaria morbidity in western Kenya. Am J Trop Med Hyg. 2003;68:121–127. - PubMed
-
- Protopopoff N, et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two fact. Lancet. 2018;391:1577–1588. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

