Use of Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer: A Systematic Review and Meta-analysis
- PMID: 31876895
- PMCID: PMC6990765
- DOI: 10.1001/jamaoncol.2019.5367
Use of Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer: A Systematic Review and Meta-analysis
Abstract
Importance: Immune checkpoint inhibitors of programmed cell death 1 (PD-1) and its ligand (PD-L1) have led to a paradigm shift in cancer treatment. Understanding the clinical efficacy and safety profile of these drugs is necessary for treatment strategy in clinical practice.
Objective: To assess the differences between anti-PD-1 and anti-PD-L1 regarding efficacy and safety shown in randomized clinical trials across various tumor types.
Data sources: Systematic searches of PubMed, Cochrane CENTRAL, and Embase were conducted from January 1, 2000, to March 1, 2019. In addition, abstracts and presentations from all major conference proceedings were reviewed.
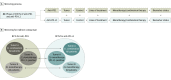
Study selection: All randomized clinical trials that compared anti-PD-1 and anti-PD-L1 with standard treatment in patients with cancer were selected as candidates. Retrospective studies, single-arm phase 1/2 studies, and trials comparing anti-PD-1 and anti-PD-L1 with other immunotherapies were excluded. Studies of anti-PD-1 and anti-PD-L1 therapy were screened and paired by the matching of clinical characteristics as mirror groups.
Data extraction and synthesis: Three investigators independently extracted data from each study following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guideline. Trial names, first author, year of publication, study design, National Clinical Trial identifier number, blinding status, study phase, pathologic characteristics, number of patients, patients' age and sex distribution, Eastern Cooperative Oncology Group Performance Status, lines of treatment, study drugs, biomarker status, follow-up time, incidence of adverse events, and hazard ratios (HRs) with 95% CIs for overall survival and progression-free survival were extracted. A random-effects model was applied for data analysis.
Main outcomes and measures: Differences in OS between anti-PD-1 and anti-PD-L1 across different cancer types were assessed. An effect size was derived from each mirror group and then pooled across all groups using a random-effects model.
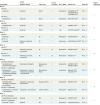
Results: Nineteen randomized clinical trials involving 11 379 patients were included in the meta-analysis. Overall, anti-PD-1 exhibited superior overall survival (HR, 0.75; 95% CI, 0.65-0.86; P < .001) and progression-free survival (HR, 0.73; 95% CI, 0.56-0.96; P = .02) compared with anti-PD-L1. No significant difference was observed in their safety profiles. Sensitivity analysis presented consistency in the overall estimates across these analyses. Consistent results were observed through frequentist and bayesian approaches with the same studies.
Conclusions and relevance: Comprehensive analysis suggests that anti-PD-1 exhibited favorable survival outcomes and a safety profile comparable to that of anti-PD-L1, which may provide a useful guide for clinicians.
Conflict of interest statement
Figures


Comment in
-
Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer-Reply.JAMA Oncol. 2020 Jul 1;6(7):1116-1117. doi: 10.1001/jamaoncol.2020.0646. JAMA Oncol. 2020. PMID: 32352479 No abstract available.
-
Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer.JAMA Oncol. 2020 Jul 1;6(7):1115-1116. doi: 10.1001/jamaoncol.2020.0637. JAMA Oncol. 2020. PMID: 32352489 No abstract available.
-
Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer.JAMA Oncol. 2020 Jul 1;6(7):1115. doi: 10.1001/jamaoncol.2020.0631. JAMA Oncol. 2020. PMID: 32352495 No abstract available.
-
Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer.JAMA Oncol. 2020 Jul 1;6(7):1114-1115. doi: 10.1001/jamaoncol.2020.0628. JAMA Oncol. 2020. PMID: 32352499 No abstract available.
-
Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer.JAMA Oncol. 2020 Jul 1;6(7):1113-1114. doi: 10.1001/jamaoncol.2020.0625. JAMA Oncol. 2020. PMID: 32352500 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

