Endocardial ventricular pulsed field ablation: a proof-of-concept preclinical evaluation
- PMID: 31876913
- PMCID: PMC7058968
- DOI: 10.1093/europace/euz341
Endocardial ventricular pulsed field ablation: a proof-of-concept preclinical evaluation
Abstract
Aims: Pulsed field ablation (PFA) is a novel, non-thermal modality that selectively ablates myocardium with ultra-short electrical impulses while sparing collateral tissues. In a proof-of-concept study, the safety and feasibility of ventricular PFA were assessed using a prototype steerable, endocardial catheter.
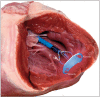
Methods and results: Under general anaesthesia, the left and right ventricles of four healthy swine were ablated using the 12-Fr deflectable PFA catheter and a deflectable sheath guided by electroanatomic mapping. Using the study catheter, electrograms were recorded for each site and pre-ablation and post-ablation pacing thresholds (at 2.0 ms pulse width) were recorded in two of four animals. After euthanasia at 35.5 days, the hearts were submitted for histology. The PFA applications (n = 39) resulted in significant electrogram reduction without ventricular arrhythmias. In ablation sites where it was measured, the pacing thresholds increased by >16.8 mA in the right ventricle (3 sites) and >16.1 mA in the left ventricle (7 sites), with non-capture at maximum amplitude (20 mA) observable in 8 of 10 sites. Gross measurements, available for 28 of 30 ablation sites, revealed average lesion dimensions to be 6.5 ± 1.7 mm deep by 22.6 ± 4.1 mm wide, with a maximum depth and width of 9.4 mm and 28.6 mm, respectively. In the PFA lesions, fibrous tissue homogeneously replaced myocytes with a narrow zone of surrounding myocytolysis and no overlying thrombus. When present, nerve fascicles and vasculature were preserved within surrounding fibrosis.
Conclusion: We demonstrate that endocardial PFA can be focally delivered using this prototype catheter to create homogeneous, myocardium-specific lesions.
Keywords: Catheter ablation; Electroporation; Pulsed field ablation; Ventricle; Ventricular tachycardia.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures





Comment in
-
Witnessing the birth of the future's ablation therapy?Europace. 2020 Mar 1;22(3):340-341. doi: 10.1093/europace/euaa019. Europace. 2020. PMID: 32031613 No abstract available.
References
-
- Davalos RV, Mir IL, Rubinsky B.. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005;33:223–31. - PubMed
-
- Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B.. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng 2006;53:1409–15. - PubMed
-
- Rubinsky B, Onik G, Mikus P.. Irreversible electroporation: a new ablation modality—clinical implications. Technol Cancer Res Treat 2007;6:37–48. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

