Impact of the change in the antitubercular regimen from three to four drugs on cure and frequency of adverse reactions in tuberculosis patients from Brazil: A retrospective cohort study
- PMID: 31877199
- PMCID: PMC6932797
- DOI: 10.1371/journal.pone.0227101
Impact of the change in the antitubercular regimen from three to four drugs on cure and frequency of adverse reactions in tuberculosis patients from Brazil: A retrospective cohort study
Abstract
Background: The Ministry of Health in Brazil included ethambutol in the intensive phase of sensible tuberculosis (TB) treatment in March 2010, due to the increasing drug resistance, and implemented the fixed dose combination in the TB treatment guidelines.
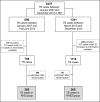
Methods: A retrospective cohort study was performed to determine the impact of change from three to four drugs schemes on the TB cure and frequency of adverse drug reactions (ADRs) in TB patients. To answer this question, we used data from 730 randomly selected patients who received anti-TB treatment between January 2007 and December 2014 in a reference center from Salvador, Brazil.
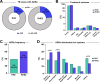
Findings: TB patients who received the RHEZ regimen (n = 365) developed ADRs more frequently than those treated with the RHZ (n = 365) (86 [23.6%] vs. 55 [15.1%]; p = 0.01). This difference in ADR incidence was even higher in patients above 30 years-old (64 [74.4%] vs. 36 [65.5%]; p = 0.01). The overall number of ADR episodes was greater in patients from the RHEZ group than in the group that received RHZ (170 [61.4%] vs. 107 [38.6%]; p = 0.03). Multivariable logistic regression analysis adjusted for age, alcohol use and diabetes demonstrated that patients receiving the RHEZ regimen had increased odds of developing ADRs than those undertaking the RHZ scheme (odds ratio [OR]: 1.61, 95% confidence interval [CI]: 1.10-2.35; p = 0.015). The overall cure rate was similar between the distinct treatment groups.
Conclusion: The patients treated with the four-drug regimen exhibited increased risk of ADRs compared to those who received the three-drug regimen, and especially in patients older than 30 years of age.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- World Health Organization Global Tuberculosis Programme G. Global Tuberculosis Report 2016. Geneva; 2016.
-
- International Union Against Tuberculosis and Lung Diseases (IUATLD), WHO Tuberculosis Programme. The promise and reality of fixed-dose combinations with rifampicin. Int Union Against Tuberc Lung Dis. 1994;69: 219–220. - PubMed
-
- World Health Organization Global Tuberculosis Programme G. Treatment of Tuberculosis Guidelines for National Programmes [Internet]. 2003 [cited 19 Sep 2016]. Available: http://apps.who.int/iris/bitstream/10665/67890/1/WHO_CDS_TB_2003.313_eng...
-
- World Health Organization / International Union Against Tuberculosis and Lung Disease Global Project on Anti-tuberculosis Drug Resistance Surveillence 1994–1997. Anti-Tuberculosis Drug Resistance in the World Report No. 1. Geneva; 1997.
-
- World Health Organization Global Tuberculosis Programme G. Global Tuberculosis Report 2009. Geneva; 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

