Non-redox cycling mechanisms of oxidative stress induced by PM metals
- PMID: 31877355
- PMCID: PMC7803379
- DOI: 10.1016/j.freeradbiomed.2019.12.027
Non-redox cycling mechanisms of oxidative stress induced by PM metals
Abstract
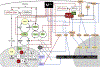
Metallic compounds contribute to the oxidative stress of ambient particulate matter (PM) exposure. The toxicity of redox inert ions of cadmium, mercury, lead and zinc, as well as redox-active ions of vanadium and chromium is underlain by dysregulation of mitochondrial function and loss of signaling quiescence. Central to the initiation of these effects is the interaction of metal ions with cysteinyl thiols on glutathione and key regulatory proteins, which leads to impaired mitochondrial electron transport and persistent pan-activation of signal transduction pathways. The mitochondrial and signaling effects are linked by the production of H2O2, generated from mitochondrial superoxide anion or through the activation of NADPH oxidase, which extends the range and amplifies the magnitude of the oxidative effects of the metals. This oxidative burden can be further potentiated by inhibitory effects of the metals on the enzymes of the glutathione and thioredoxin systems. Along with the better-known Fenton-based mechanisms, the non-redox cycling mechanisms of oxidative stress induced by metals constitute significant pathways for cellular injury induced by PM inhalation.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest None.
Figures
References
-
- Strak M, Janssen NA, Godri KJ, Gosens I, Mudway IS, Cassee FR, Lebret E, Kelly FJ, Harrison RM, Brunekreef B, Steenhof M, Hoek G, Respiratory health effects of airborne particulate matter: the role of particle size, composition, and oxidative potential-the RAPTES project, Environ. Health Perspect. 120 (2012) 1183–1189. - PMC - PubMed
-
- Chen LC, Lippmann M, Effects of metals within ambient air particulate matter (PM) on human health, Inhal. Toxicol. 21 (2009) 1–31. - PubMed
-
- Kelly FJ, Fussell JC, Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter, Atmos. Environ. 60 (2012) 504–526.
-
- Malandrino M, Casazza M, Abollino O, Minero C, Maurino V, Size resolved metal distribution in the PM matter of the city of Turin (Italy), Chemosphere 147 (2016) 477–489. - PubMed
-
- Song F, Gao Y, Size Distributions of Trace Elements Associated with Ambient Particular Matter in the Affinity of a Major Highway in the New Jersey-New York Metropolitan Area Atmospheric Environment vol. 45, (2011), pp. 6714–6723.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

