Synergistic Effects of Nanomedicine Targeting TNFR2 and DNA Demethylation Inhibitor-An Opportunity for Cancer Treatment
- PMID: 31877663
- PMCID: PMC7016661
- DOI: 10.3390/cells9010033
Synergistic Effects of Nanomedicine Targeting TNFR2 and DNA Demethylation Inhibitor-An Opportunity for Cancer Treatment
Abstract
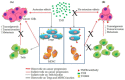
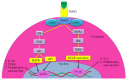
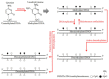
Tumor necrosis factor receptor 2 (TNFR2) is expressed on some tumor cells, such as myeloma, Hodgkin lymphoma, colon cancer and ovarian cancer, as well as immunosuppressive cells. There is increasingly evidence that TNFR2 expression in cancer microenvironment has significant implications in cancer progression, metastasis and immune evasion. Although nanomedicine has been extensively studied as a carrier of cancer immunotherapeutic agents, no study to date has investigated TNFR2-targeting nanomedicine in cancer treatment. From an epigenetic perspective, previous studies indicate that DNA demethylation might be responsible for high expressions of TNFR2 in cancer models. This perspective review discusses a novel therapeutic strategy based on nanomedicine that has the capacity to target TNFR2 along with inhibition of DNA demethylation. This approach may maximize the anti-cancer potential of nanomedicine-based immunotherapy and, consequently, markedly improve the outcomes of the management of patients with malignancy.
Keywords: TNF; immunosuppressive; immunotherapy; nanoparticles; regulatory T cells.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

