Effects of Gangliosides on Spermatozoa, Oocytes, and Preimplantation Embryos
- PMID: 31877897
- PMCID: PMC6982094
- DOI: 10.3390/ijms21010106
Effects of Gangliosides on Spermatozoa, Oocytes, and Preimplantation Embryos
Abstract
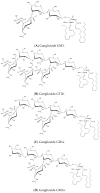
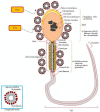
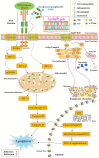
Gangliosides are sialic acid-containing glycosphingolipids, which are the most abundant family of glycolipids in eukaryotes. Gangliosides have been suggested to be important lipid molecules required for the control of cellular procedures, such as cell differentiation, proliferation, and signaling. GD1a is expressed in interstitial cells during ovarian maturation in mice and exogenous GD1a is important to oocyte maturation, monospermic fertilization, and embryonic development. In this context, GM1 is known to influence signaling pathways in cells and is important in sperm-oocyte interactions and sperm maturation processes, such as capacitation. GM3 is expressed in the vertebrate oocyte cytoplasm, and exogenously added GM3 induces apoptosis and DNA injury during in vitro oocyte maturation and embryogenesis. As a consequence of this, ganglioside GT1b and GM1 decrease DNA fragmentation and act as H2O2 inhibitors on germ cells and preimplantation embryos. This review describes the functional roles of gangliosides in spermatozoa, oocytes, and early embryonic development.
Keywords: apoptosis; embryo; ganglioside; maturation; oocyte; spermatozoa.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Mclntosh T.J. Overview of membrane rafts. Meth. Mol. Biol. 2007;398:1–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

