Switching to a Healthy Diet Prevents the Detrimental Effects of Western Diet in a Colitis-Associated Colorectal Cancer Model
- PMID: 31877961
- PMCID: PMC7019913
- DOI: 10.3390/nu12010045
Switching to a Healthy Diet Prevents the Detrimental Effects of Western Diet in a Colitis-Associated Colorectal Cancer Model
Abstract
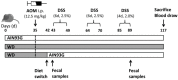
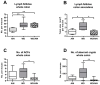
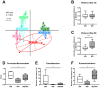
Inflammatory bowel disease increases the odds of developing colitis-associated cancer. We hypothesized that Western-style diet (WD) aggravates azoxymethane (AOM)/dextran sulfate sodium salt (DSS)-induced colitis-associated tumorigenesis and that switching to the standard AIN93G diet will ameliorate disease symptoms even after cancer initiation. Female BALB/c mice received either WD (WD group) or standard AIN93G diet (AIN group) for the whole experimental period. After five weeks, the mice received 12.5 mg/kg AOM intraperitoneally, followed by three DSS cycles. In one group of mice, the WD was switched to AIN93G the day before starting the first DSS cycle (WD/AIN group). Feeding the WD during the whole experimental period aggravated colitis symptoms, shortened the colon (p < 0.05), changed microbiota composition and increased tumor promotion. On molecular level, the WD reduced proliferation (p < 0.05) and increased expression of the vitamin D catabolizing enzyme Cyp24a1 (p < 0.001). The switch to the AIN93G diet ameliorated this effect, reflected by longer colons, fewer (p < 0.05) and smaller (p < 0.01) aberrant colonic crypt foci, comparable with the AIN group. Our results show that switching to a healthy diet, even after cancer initiation is able to revert the deleterious effect of the WD and could be an effective preventive strategy to reduce colitis symptoms and prevent tumorigenesis.
Keywords: CYP24A1; Wnt pathway; aberrant crypt foci; colitis-associated cancer; inflammatory bowel disease; microbiome; mucosal regeneration; non-alcoholic fatty liver disease; vitamin D; western diet.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Triantafillidis J.K., Nasioulas G., Kosmidis P.A. Colorectal cancer and inflammatory bowel disease: Epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727–2737. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

