Benefits of the Use of Lactic Acid Bacteria Starter in Green Cracked Cypriot Table Olives Fermentation
- PMID: 31878011
- PMCID: PMC7023104
- DOI: 10.3390/foods9010017
Benefits of the Use of Lactic Acid Bacteria Starter in Green Cracked Cypriot Table Olives Fermentation
Abstract
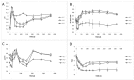
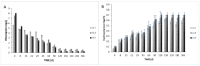
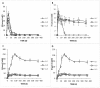
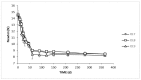
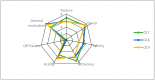
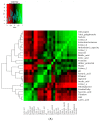
Table olives are one of the most established Mediterranean vegetables, having an exponential increase consumption year by year. In the natural-style processing, olives are produced by spontaneous fermentation, without any chemical debittering. This natural fermentation process remains empirical and variable since it is strongly influenced by physicochemical parameters and microorganism presence in olive drupes. In the present work, Cypriot green cracked table olives were processed directly in brine (natural olives), using three distinct methods: spontaneous fermentation, inoculation with lactic acid bacteria at a 7% or a 10% NaCl concentration. Sensory, physicochemical, and microbiological alterations were monitored at intervals, and major differences were detected across treatments. Results indicated that the predominant microorganisms in the inoculated treatments were lactic acid bacteria, while yeasts predominated in control. As a consequence, starter culture contributed to a crucial effect on olives fermentation, leading to faster acidification and lower pH. This was attributed to a successful lactic acid fermentation, contrasting the acetic and alcoholic fermentation observed in control. Furthermore, it was established that inhibition of enterobacteria growth was achieved in a shorter period and at a significantly lower salt concentration, compared to the spontaneous fermentation. Even though no significant variances were detected in terms of the total phenolic content and antioxidant capacity, the degradation of oleuropein was achieved faster in inoculated treatments, thus, producing higher levels of hydroxytyrosol. Notably, the reduction of salt concentration, in combination with the use of starter, accented novel organoleptic characteristics in the final product, as confirmed from a sensory panel; hence, it becomes obvious that the production of Cypriot table olives at reduced NaCl levels is feasible.
Keywords: fermentation; microbiological changes; organoleptic; physicochemical; table olives.
Conflict of interest statement
None of the authors has a financial or personal relationship with other people or organizations that could inappropriately influence or bias this publication.
Figures




References
-
- (IOC), I.O.O.C. Updates Series of World Statistics on Production, Imports, Exports and Consumption. [(accessed on 11 November 2019)];2018 Available online: https://www.internationaloliveoil.org/wp-content/uploads/2019/11/product....
-
- Bleve G., Tufariello M., Durante M., Perbellini E., Ramires F.A., Grieco F., Cappello M.S., de Domenico S., Mita G., Tasioula-Margari M., et al. Physico-chemical and microbiological characterization of spontaneous fermentation of Cellina di Nardò and Leccino table olives. Front. Microbiol. 2014;5:1–18. doi: 10.3389/fmicb.2014.00570. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

