Changes in Energy Reserves and Gene Expression Elicited by Freezing and Supercooling in the Antarctic Midge, Belgica antarctica
- PMID: 31878219
- PMCID: PMC7022800
- DOI: 10.3390/insects11010018
Changes in Energy Reserves and Gene Expression Elicited by Freezing and Supercooling in the Antarctic Midge, Belgica antarctica
Abstract
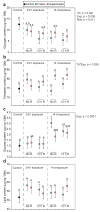
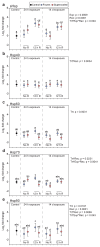
Freeze-tolerance, or the ability to survive internal ice formation, is relatively rare among insects. Larvae of the Antarctic midge Belgica antarctica are freeze-tolerant year-round, but in dry environments, the larvae can remain supercooled (i.e., unfrozen) at subzero temperatures. In previous work with summer-acclimatized larvae, we showed that freezing is considerably more stressful than remaining supercooled. Here, these findings are extended by comparing survival, tissue damage, energetic costs, and stress gene expression in larvae that have undergone an artificial winter acclimation regime and are either frozen or supercooled at -5 °C. In contrast to summer larvae, winter larvae survive at -5 °C equally well for up to 14 days, whether frozen or supercooled, and there is no tissue damage at these conditions. In subsequent experiments, we measured energy stores and stress gene expression following cold exposure at -5 °C for either 24 h or 14 days, with and without a 12 h recovery period. We observed slight energetic costs to freezing, as frozen larvae tended to have lower glycogen stores across all groups. In addition, the abundance of two heat shock protein transcripts, hsp60 and hsp90, tended to be higher in frozen larvae, indicating higher levels of protein damage following freezing. Together, these results indicate a slight cost to being frozen relative to remaining supercooled, which may have implications for the selection of hibernacula and responses to climate change.
Keywords: Antarctica; Belgica antarctica; energy stores; freeze-tolerance; heat shock proteins.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript or in the decision to publish the results.
Figures

References
-
- Lee R.E. Insect cold-hardiness—To freeze or not to freeze—How insects survive low temperatures. Bioscience. 1989;39:308–313. doi: 10.2307/1311113. - DOI
-
- Lee R.E. A primer on insect cold tolerance. In: Denlinger D.L., Lee R.E., editors. Low Temperature Biology of Insects. Cambridge University Press; Cambridge, UK: 2010. pp. 3–34.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

