AtKATANIN1 Modulates Microtubule Depolymerization and Reorganization in Response to Salt Stress in Arabidopsis
- PMID: 31878228
- PMCID: PMC6981882
- DOI: 10.3390/ijms21010138
AtKATANIN1 Modulates Microtubule Depolymerization and Reorganization in Response to Salt Stress in Arabidopsis
Abstract
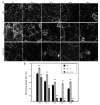
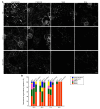
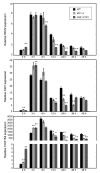
The microtubule cytoskeleton is a dynamic system that plays vital roles in fundamental cellular processes and in responses to environmental stumili. Salt stress induced depolymerization and reorganization of microtubules are believed to function in the promotion of survival in Arabidopsis. Microtubule-severing enzyme ATKATANIN1 (AtKTN1) is recognized as a MAP that help to maintain organized microtubule structure. To date, whether AtKTN1 is involved in response to salt stress in Arabidopsis remains unknown. Here, our phenotypic analysis showed that the overexpression of AtKTN1 decreased tolerance to salt stress, whereas the knock-out of AtKTN1 increased salt tolerance in the early stage but decreased salt tolerance in the later stage. Microscopic analysis revealed that microtubule organization and dynamics are distorted in both overexpression and mutant cells which, in turn, resulted in an abnormal disassembly and reorganization under salt stress. Moreover, qRT analysis revealed that stress-responsive genes were down-regulated in overexpression and mutant cells compared to WT cells under salt stress. Taken together, our results indicated roles of AtKTN1 in modulating microtubule organization, salt-stress induced microtubule disruption and recovery, and its involvement in stress-related signaling pathways.
Keywords: Arabidopsis; KATANIN1; dynamics; microtubule; organization; salt stress.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

