Flavescence Dorée Phytoplasma Has Multiple ftsH Genes that Are Differentially Expressed in Plants and Insects
- PMID: 31878312
- PMCID: PMC6981957
- DOI: 10.3390/ijms21010150
Flavescence Dorée Phytoplasma Has Multiple ftsH Genes that Are Differentially Expressed in Plants and Insects
Abstract
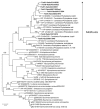
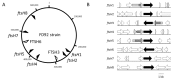
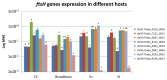
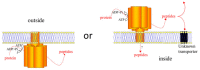
Flavescence dorée (FD) is a severe epidemic disease of grapevines caused by FD phytoplasma (FDP) transmitted by the leafhopper vector Scaphoideus titanus. The recent sequencing of the 647-kbp FDP genome highlighted an unusual number of genes encoding ATP-dependent zinc proteases FtsH, which have been linked to variations in the virulence of "Candidatus Phytoplasma mali" strains. The aims of the present study were to predict the FtsH repertoire of FDP, to predict the functional domains and topologies of the encoded proteins in the phytoplasma membrane and to measure the expression profiles in different hosts. Eight complete ftsH genes have been identified in the FDP genome. In addition to ftsH6, which appeared to be the original bacterial ortholog, the other seven gene copies were clustered on a common distinct phylogenetic branch, suggesting intra-genome duplication of ftsH. The expression of these proteins, quantified in plants and insect vectors in natural and experimental pathosystems, appeared to be modulated in a host-dependent manner. Two of the eight FtsH C-tails were predicted by Phobius software to be extracellular and, therefore, in direct contact with the host cellular content. As phytoplasmas cannot synthesize amino acids, our data raised questions regarding the involvement of FtsH in the adaptation to hosts via potentially enhanced recycling of phytoplasma cellular proteins and host protein degradation.
Keywords: membrane proteases; phytoplasmas; protein metabolism; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Genetic Diversity of Flavescence Dorée Phytoplasmas at the Vineyard Scale.Appl Environ Microbiol. 2019 May 2;85(10):e03123-18. doi: 10.1128/AEM.03123-18. Print 2019 May 15. Appl Environ Microbiol. 2019. PMID: 30877117 Free PMC article.
-
Heterologous expression and processing of the flavescence dorée phytoplasma variable membrane protein VmpA in Spiroplasma citri.BMC Microbiol. 2015 Apr 2;15:82. doi: 10.1186/s12866-015-0417-5. BMC Microbiol. 2015. PMID: 25879952 Free PMC article.
-
Natterin-like and legumain insect gut proteins promote the multiplication of a vector-borne bacterial plant pathogen.Microbiol Res. 2025 Feb;291:127984. doi: 10.1016/j.micres.2024.127984. Epub 2024 Nov 28. Microbiol Res. 2025. PMID: 39616656
-
Immunodominant membrane proteins of phytoplasmas.Microbiology (Reading). 2016 Aug;162(8):1267-1273. doi: 10.1099/mic.0.000331. Epub 2016 Jul 5. Microbiology (Reading). 2016. PMID: 27384683 Review.
-
Current view on phytoplasma genomes and encoded metabolism.ScientificWorldJournal. 2012;2012:185942. doi: 10.1100/2012/185942. Epub 2011 Dec 5. ScientificWorldJournal. 2012. PMID: 22550465 Free PMC article. Review.
Cited by
-
Host-Pathogen Interaction 3.0.Int J Mol Sci. 2022 Oct 24;23(21):12811. doi: 10.3390/ijms232112811. Int J Mol Sci. 2022. PMID: 36361600 Free PMC article.
-
Competitive Exclusion of Flavescence dorée Phytoplasma Strains in Catharanthus roseus Plants.Plants (Basel). 2020 Nov 17;9(11):1594. doi: 10.3390/plants9111594. Plants (Basel). 2020. PMID: 33213006 Free PMC article.
-
The Complete Genome of the "Flavescence Dorée" Phytoplasma Reveals Characteristics of Low Genome Plasticity.Biology (Basel). 2022 Jun 23;11(7):953. doi: 10.3390/biology11070953. Biology (Basel). 2022. PMID: 36101334 Free PMC article.
-
Competition among Flavescence Dorée Phytoplasma Strains in the Experimental Insect Vector Euscelidius variegatus.Insects. 2023 Jun 23;14(7):575. doi: 10.3390/insects14070575. Insects. 2023. PMID: 37504582 Free PMC article.
-
SEOR2 in Arabidopsis mediates Ca2+ dependent defense against phytoplasmas and reduction of plant growth.Sci Rep. 2025 May 22;15(1):17829. doi: 10.1038/s41598-025-01374-8. Sci Rep. 2025. PMID: 40404713 Free PMC article.
References
-
- Boudon-Padieu E. Flavescence dorée of the grapevine, knowledge and new developments in epidemiology, etiology and diagnosis. ATTI Giornate Fitopatol. 2002;1:15–34.
-
- EFSA Panel on Plant Health (PLH) Scientific Opinion on pest categorisation of Grapevine Flavescence dorée: Grapevine Flavescence dorée pest categorisation. EFSA J. 2014;12:3851. doi: 10.2903/j.efsa.2014.3851. - DOI
-
- Schvester D., Carle P., Moutous G. Sur la transmission de la flavescence dorée des vignes par une cicadelle. C. R. Acad. Agric. Fr. 1961;47:1021–1024.
-
- Caudwell A., Giannotti J., Kuszala C., Larrue J. Etude du rôle de particules de type “mycoplasme” dans l’étiologie de la flavescence dorée de la vigne. Examen cytologique des plantes. Ann. Phytopathol. 1971;3:107–123.
-
- Caudwell A. Deux années d’étude sur la flavescence dorée, nouvelle maladie grave de la vigne. Ann. Amélior. Plantes. 1957;4:359–363.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

