Use of a flexible optical fibre bundle to interrogate a Fabry-Perot sensor for photoacoustic imaging
- PMID: 31878562
- PMCID: PMC7046039
- DOI: 10.1364/OE.27.037886
Use of a flexible optical fibre bundle to interrogate a Fabry-Perot sensor for photoacoustic imaging
Abstract
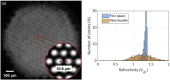
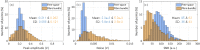
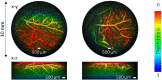
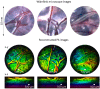
Photoacoustic imaging systems based on a Fabry Perot (FP) ultrasound sensor that is read-out by scanning a free-space laser beam over its surface can provide high resolution photoacoustic images. However, this type of free-space scanning usually requires a bulky 2-axis galvanometer based scanner that is not conducive to the realization of a lightweight compact imaging head. It is also unsuitable for endoscopic applications that may require complex and flexible access. To address these limitations, the use of a flexible, coherent fibre bundle to interrogate the FP sensor has been investigated. A laboratory set-up comprising an x-y scanner, a commercially available, 1.35 mm diameter, 18,000 core flexible fibre bundle with a custom-designed telecentric optical relay at its distal end was used. Measurements of the optical and acoustic performance of the FP sensor were made and compared to that obtained using a conventional free-space FP based scanner. Spatial variations in acoustic sensitivity were greater and the SNR lower with the fibre bundle implementation but high quality photoacoustic images could still be obtained. 3D images of phantoms and ex vivo tissues with a spatial resolution and fidelity consistent with a free-space scanner were acquired. By demonstrating the feasibility of interrogating the FP sensor with a flexible fibre bundle, this study advances the realization of compact hand-held clinical scanners and flexible endoscopic devices based on the FP sensing concept.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Oraevsky A. A., Karabutov A. A., “Optoacoustic tomography,” Biomed. Photonics Handbook 34, 1–34 (2003).10.1201/9780203008997.ch34 - DOI
-
- Laufer J., Delpy D., Elwell C., Beard P., “Quantitative spatially resolved measurement of tissue chromophore concentrations using photoacoustic spectroscopy: application to the measurement of blood oxygenation and haemoglobin concentration,” Phys. Med. Biol. 52(1), 141–168 (2007).10.1088/0031-9155/52/1/010 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

