Therapeutic Myeloperoxidase Inhibition Attenuates Neutrophil Activation, ANCA-Mediated Endothelial Damage, and Crescentic GN
- PMID: 31879336
- PMCID: PMC7003306
- DOI: 10.1681/ASN.2019060618
Therapeutic Myeloperoxidase Inhibition Attenuates Neutrophil Activation, ANCA-Mediated Endothelial Damage, and Crescentic GN
Abstract
Background: Myeloperoxidase released after neutrophil and monocyte activation can generate reactive oxygen species, leading to host tissue damage. Extracellular glomerular myeloperoxidase deposition, seen in ANCA-associated vasculitis, may enhance crescentic GN through antigen-specific T and B cell activation. Myeloperoxidase-deficient animals have attenuated GN early on, but augmented T cell responses. We investigated the effect of myeloperoxidase inhibition, using the myeloperoxidase inhibitor AZM198, to understand its potential role in treating crescentic GN.
Methods: We evaluated renal biopsy samples from patients with various forms of crescentic GN for myeloperoxidase and neutrophils, measured serum myeloperoxidase concentration in patients with ANCA-associated vasculitis and controls, and assessed neutrophil extracellular trap formation, reactive oxygen species production, and neutrophil degranulation in ANCA-stimulated neutrophils in the absence and presence of AZM198. We also tested the effect of AZM198 on ANCA-stimulated neutrophil-mediated endothelial cell damage in vitro, as well as on crescentic GN severity and antigen-specific T cell reactivity in the murine model of nephrotoxic nephritis.
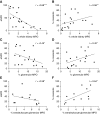
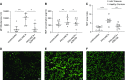
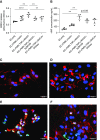
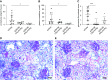
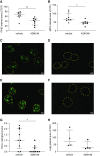
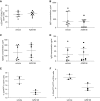
Results: All biopsy specimens with crescentic GN had extracellular glomerular myeloperoxidase deposition that correlated significantly with eGFR and crescent formation. In vitro, AZM198 led to a significant reduction in neutrophil extracellular trap formation, reactive oxygen species production, and released human neutrophil peptide levels, and attenuated neutrophil-mediated endothelial cell damage. In vivo, delayed AZM198 treatment significantly reduced proteinuria, glomerular thrombosis, serum creatinine, and glomerular macrophage infiltration, without increasing adaptive T cell responses.
Conclusions: Myeloperoxidase inhibition reduced neutrophil degranulation and neutrophil-mediated endothelial cell damage in patients with ANCA-associated vasculitis. In preclinical crescentic GN, delayed myeloperoxidase inhibition suppressed kidney damage without augmenting adaptive immune responses, suggesting it might offer a novel adjunctive therapeutic approach in crescentic GN.
Keywords: ANCA; glomerular endothelial cells; glomerulonephritis; myeloperoxidase; neutrophils; vasculitis.
Copyright © 2020 by the American Society of Nephrology.
Figures



References
-
- Klebanoff SJ: Myeloperoxidase: Friend and foe. J Leukoc Biol 77: 598–625, 2005 - PubMed
-
- Bradley PP, Christensen RD, Rothstein G: Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 60: 618–622, 1982 - PubMed
-
- Dinauer MC: Disorders of neutrophil function: An overview. Methods Mol Biol 1124: 501–515, 2014 - PubMed
-
- Winterbourn CC: Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology 181-182: 223–227, 2002 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

