The frequency of cortical microstimulation shapes artificial touch
- PMID: 31879342
- PMCID: PMC6969512
- DOI: 10.1073/pnas.1916453117
The frequency of cortical microstimulation shapes artificial touch
Abstract
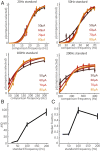
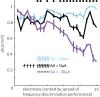
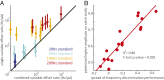
Intracortical microstimulation (ICMS) of the somatosensory cortex evokes vivid tactile sensations and can be used to convey sensory feedback from brain-controlled bionic hands. Changes in ICMS frequency lead to changes in the resulting sensation, but the discriminability of frequency has only been investigated over a narrow range of low frequencies. Furthermore, the sensory correlates of changes in ICMS frequency remain poorly understood. Specifically, it remains to be elucidated whether changes in frequency only modulate sensation magnitude-as do changes in amplitude-or whether they also modulate the quality of the sensation. To fill these gaps, we trained monkeys to discriminate the frequency of ICMS pulse trains over a wide range of frequencies (from 10 to 400 Hz). ICMS amplitude also varied across stimuli to dissociate sensation magnitude from ICMS frequency and ensure that animals could not make frequency judgments based on magnitude. We found that animals could consistently discriminate ICMS frequency up to ∼200 Hz but that the sensory correlates of frequency were highly electrode dependent: On some electrodes, changes in frequency were perceptually distinguishable from changes in amplitude-seemingly giving rise to a change in sensory quality; on others, they were not. We discuss the implications of our findings for neural coding and for brain-controlled bionic hands.
Keywords: artificial touch; bionic hands; neuroprosthetics; sensory feedback; temporal coding.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Berg J. A., et al. , Behavioral demonstration of a somatosensory neuroprosthesis. IEEE Trans. Neural Syst. Rehabil. Eng. 21, 500–507 (2013). - PubMed
-
- Flesher S. N., et al. , Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med. 8, 361ra141 (2016). - PubMed
-
- Flesher S., et al. , “Restoring touch through intracortical microstimulation of human somatosensory cortex” in Proceedings of 2017 New Generation of CAS (NGCAS) (IEEE, 2017), pp. 185–188.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

