CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas
- PMID: 31879345
- PMCID: PMC6969504
- DOI: 10.1073/pnas.1910856117
CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas
Abstract
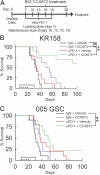
Immunotherapy directed at the PD-L1/PD-1 axis has produced treatment advances in various human cancers. Unfortunately, progress has not extended to glioblastoma (GBM), with phase III clinical trials assessing anti-PD-1 monotherapy failing to show efficacy in newly diagnosed and recurrent tumors. Myeloid-derived suppressor cells (MDSCs), a subset of immunosuppressive myeloid derived cells, are known to infiltrate the tumor microenvironment of GBM. Growing evidence suggests the CCL2-CCR2 axis is important for this process. This study evaluated the combination of PD-1 blockade and CCR2 inhibition in anti-PD-1-resistant gliomas. CCR2 deficiency unmasked an anti-PD-1 survival benefit in KR158 glioma-bearing mice. CD11b+/Ly6Chi/PD-L1+ MDSCs within established gliomas decreased with a concomitant increase in overall CCR2+ cells and MDSCs within bone marrow of CCR2-deficient mice. The CCR2 antagonist CCX872 increased median survival as a monotherapy in KR158 glioma-bearing animals and further increased median and overall survival when combined with anti-PD-1. Additionally, combination of CCX872 and anti-PD-1 prolonged median survival time in 005 GSC GBM-bearing mice. In both models, CCX872 decreased tumor associated MDSCs and increased these cells within the bone marrow. Examination of tumor-infiltrating lymphocytes revealed an elevated population, increased IFNγ expression, indicating enhanced cytolytic activity, as well as decreased expression of exhaustion markers in CD4+ and CD8+ T cells following combination treatment. These data establish that combining CCR2 and PD-1 blockade extends survival in clinically relevant murine glioma models and provides the basis on which to advance this combinatorial treatment toward early-phase human trials.
Keywords: glioblastoma; immunotherapy; myeloid-derived suppressor cells; programmed death-1; tumor microenvironment.
Conflict of interest statement
Competing interest statement: J.J.C., I.F.C., R.S., and T.J.S. are all employed by ChemoCentryx and are stockholders. D.A.M. holds patented technologies that have been licensed or have exclusive options to license to Celldex Therapeutics, Annias, Immunomic Therapeutics, and iOncologi. D.A.M. receives research funding from Immunomic Therapeutics. D.A.M. serves as an advisor/consultant to Bristol-Myers Squibb, Tocagen, and Oncorus. D.A.M. is cofounder of iOncologi, Inc., an immunooncology biotechnology company. Neither any reagent nor any funding from these organizations was used in this study. J.A.F.-T., D.L., A.G., M.R.S., M.D., and J.K.H. have no competing interests to report. R.K.J. received honorarium from Amgen; consultant fees from Chugai, Merck, Ophthotech, Pfizer, SPARC, SynDevRx, XTuit; owns equity in Enlight, Ophthotech, SynDevRx; and serves on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, the Tekla Healthcare Opportunities Fund, and the Tekla World Healthcare Fund. Neither any reagent nor any funding from these organizations was used in this study.
Figures





References
-
- Stupp R., et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups ; National Cancer Institute of Canada Clinical Trials Group , Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996 (2005). - PubMed
-
- Furnari F. B., et al. , Malignant astrocytic glioma: Genetics, biology, and paths to treatment. Genes Dev. 21, 2683–2710 (2007). - PubMed
-
- Wen P. Y., Kesari S., Malignant gliomas in adults. N. Engl. J. Med. 359, 492–507 (2008). - PubMed
-
- Johansson Swartling F., Identifying candidate genes involved in brain tumor formation. Ups. J. Med. Sci. 113, 1–38 (2008). - PubMed
-
- Colman H., Aldape K., Molecular predictors in glioblastoma: Toward personalized therapy. Arch. Neurol. 65, 877–883 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

