Predictive factors for responses to primary medical treatment with lanreotide autogel 120 mg in acromegaly: post hoc analyses from the PRIMARYS study
- PMID: 31879842
- PMCID: PMC7066297
- DOI: 10.1007/s11102-019-01020-3
Predictive factors for responses to primary medical treatment with lanreotide autogel 120 mg in acromegaly: post hoc analyses from the PRIMARYS study
Abstract
Purpose: PRIMARYS (NCT00690898) was a 48-week, open-label, phase 3b study, evaluating treatment with the somatostatin receptor ligand lanreotide autogel (stable dose: 120 mg/28 days) in treatment-naïve patients with growth hormone (GH)-secreting pituitary macroadenoma. This post hoc analysis aimed to evaluate factors predictive of long-term responses.
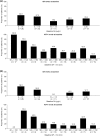
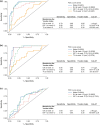
Methods: Potential predictive factors evaluated were: sex, age, and body mass index at baseline; and GH, insulin-like growth factor-1 (IGF-1), and tumor volume (TV) at baseline and week 12, using univariate regression analyses. Treatment responses were defined as hormonal control (GH ≤ 2.5 µg/L and age- and sex-normalized IGF-1), tight hormonal control (GH < 1.0 µg/L and normalized IGF-1), or ≥ 20% TV reduction (TVR). Receiver-operating-characteristic (ROC) curves were constructed using predictive factors significant in univariate analyses. Cut-off values for predicting treatment responses at 12 months were derived by maximizing the Youden index (J).
Results: At baseline, older age, female sex, and lower IGF-1 levels were associated with an increased probability of achieving long-term hormonal control. ROC area-under-the curve (AUC) values for hormonal control were high for week-12 GH and IGF-1 levels (0.87 and 0.93, respectively); associated cut-off values were 1.19 μg/L and 110% of the upper limit of normal (ULN), respectively. Results were similar for tight hormonal control (AUC values: 0.92 [GH] and 0.87 [IGF-1]; cut-off values: 1.11 μg/L and 125% ULN, respectively). AUC and J values associated with TVR were low.
Conclusions: The use of predictive factors at baseline and week 12 of treatment could inform clinical expectations of the long-term efficacy of lanreotide autogel.
Keywords: Acromegaly; Baseline IGF-1; Growth hormone; Hormonal response; Predictive factors; Somatostatin receptor ligands.
Conflict of interest statement
SP has served as an advisory board member for Ipsen and Novartis, and has received honoraria for speaking at symposia for Ipsen, Novartis and Pfizer. AH and CS are employed by Ipsen. PC has served as a consultant and speaker for Ipsen, Novartis and Pfizer, and has served as an advisory board member for Ipsen.
Figures

References
-
- Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, et al. Expert consensus document: a consensus on the medical treatment of acromegaly. Nat Rev Endocrinol. 2014;10(4):243–248. - PubMed
-
- Katznelson L, Laws ER, Jr, Melmed S, Molitch ME, Murad MH, et al. Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2014;99(11):3933–3951. - PubMed
-
- Oberg K, Lamberts SW. Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future. Endocr Relat Cancer. 2016;23(12):R551–R566. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

