Modulation of Biliary Cancer Chemo-Resistance Through MicroRNA-Mediated Rewiring of the Expansion of CD133+ Cells
- PMID: 31879968
- PMCID: PMC7590111
- DOI: 10.1002/hep.31094
Modulation of Biliary Cancer Chemo-Resistance Through MicroRNA-Mediated Rewiring of the Expansion of CD133+ Cells
Abstract
Background and aims: Changes in single microRNA (miRNA) expression have been associated with chemo-resistance in biliary tract cancers (BTCs). However, a global assessment of the dynamic role of the microRNome has never been performed to identify potential therapeutic targets that are functionally relevant in the BTC cell response to chemotherapy.
Approach and results: High-throughput screening (HTS) of 997 locked nucleic acid miRNA inhibitors was performed in six cholangiocarcinoma cell lines treated with cisplatin and gemcitabine (CG) seeking changes in cell viability. Validation experiments were performed with mirVana probes. MicroRNA and gene expression was assessed by TaqMan assay, RNA-sequencing, and in situ hybridization in four independent cohorts of human BTCs. Knockout of microRNA was achieved by CRISPR-CAS9 in CCLP cells (MIR1249KO) and tested for effects on chemotherapy sensitivity in vitro and in vivo. HTS revealed that MIR1249 inhibition enhanced chemotherapy sensitivity across all cell lines. MIR1249 expression was increased in 41% of cases in human BTCs. In validation experiments, MIR1249 inhibition did not alter cell viability in untreated or dimethyl sulfoxide-treated cells; however, it did increase the CG effect. MIR1249 expression was increased in CD133+ biliary cancer cells freshly isolated from the stem cell niche of human BTCs as well as in CD133+ chemo-resistant CCLP cells. MIR1249 modulated the chemotherapy-induced enrichment of CD133+ cells by controlling their clonal expansion through the Wnt-regulator FZD8. MIR1249KO cells had impaired expansion of the CD133+ subclone and its enrichment after chemotherapy, reduced expression of cancer stem cell markers, and increased chemosensitivity. MIR1249KO xenograft BTC models showed tumor shrinkage after exposure to weekly CG, whereas wild-type models showed only stable disease over treatment.
Conclusions: MIR1249 mediates resistance to CG in BTCs and may be tested as a target for therapeutics.
© 2020 The Authors. Hepatology published by Wiley Periodicals, Inc., on behalf of American Association for the Study of Liver Diseases.
Figures
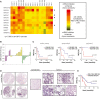

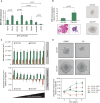

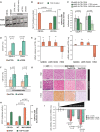

References
-
- Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor‐Robinson SD, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol 2012;56:848‐85 4. - PubMed
-
- Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol 2014;60:1268‐1289. - PubMed
-
- Marcano‐Bonilla L, Mohamed EA, Mounajjed T, Roberts LR. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol 2016;5:61. - PubMed
-
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273‐1281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

