1,25-Dihydroxyvitamin D protects against age-related osteoporosis by a novel VDR-Ezh2-p16 signal axis
- PMID: 31880094
- PMCID: PMC6996957
- DOI: 10.1111/acel.13095
1,25-Dihydroxyvitamin D protects against age-related osteoporosis by a novel VDR-Ezh2-p16 signal axis
Abstract
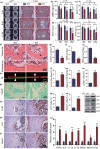
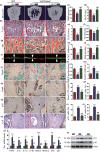
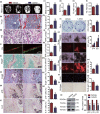
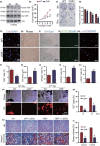
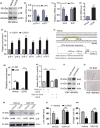
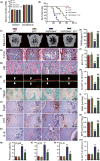
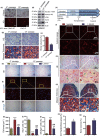
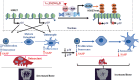
To determine whether 1,25-dihydroxyvitamin D (1,25(OH)2 D) can exert an anti-osteoporosis role through anti-aging mechanisms, we analyzed the bone phenotype of mice with 1,25(OH)2 D deficiency due to deletion of the enzyme, 25-hydroxyvitamin D 1α-hydroxylase, while on a rescue diet. 1,25(OH)2 D deficiency accelerated age-related bone loss by activating the p16/p19 senescence signaling pathway, inhibiting osteoblastic bone formation, and stimulating osteoclastic bone resorption, osteocyte senescence, and senescence-associated secretory phenotype (SASP). Supplementation of exogenous 1,25(OH)2 D3 corrected the osteoporotic phenotype caused by 1,25(OH)2 D deficiency or natural aging by inhibiting the p16/p19 pathway. The proliferation, osteogenic differentiation, and ectopic bone formation of bone marrow mesenchymal stem cells derived from mice with genetically induced deficiency of the vitamin D receptor (VDR) were significantly reduced by mechanisms including increased oxidative stress, DNA damage, and cellular senescence. We also demonstrated that p16 deletion largely rescued the osteoporotic phenotype caused by 1,25(OH)2 D3 deficiency, whereas 1,25(OH)2 D3 could up-regulate the enzyme Ezh2 via VDR-mediated transcription thereby enriching H3K27me3 and repressing p16/p19 transcription. Finally, we demonstrated that treatment with 1,25(OH)2 D3 improved the osteogenic defects of human BM-MSCs caused by repeated passages by stimulating their proliferation and inhibiting their senescence via the VDR-Ezh2-p16 axis. The results of this study therefore indicate that 1,25(OH)2 D3 plays a role in preventing age-related osteoporosis by up-regulating Ezh2 via VDR-mediated transcription, increasing H3K27me3 and repressing p16 transcription, thus promoting the proliferation and osteogenesis of BM-MSCs and inhibiting their senescence, while also stimulating osteoblastic bone formation, and inhibiting osteocyte senescence, SASP, and osteoclastic bone resorption.
Keywords: Ezh2; Vitamin D; cellular senescence; osteogenesis; osteoporosis; p16.
© 2019 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

