Correcting time-intensity curves in dynamic contrast-enhanced breast MRI for inhomogeneous excitation fields at 7T
- PMID: 31880346
- PMCID: PMC7217168
- DOI: 10.1002/mrm.28147
Correcting time-intensity curves in dynamic contrast-enhanced breast MRI for inhomogeneous excitation fields at 7T
Abstract
Purpose: Inhomogeneous excitation at ultrahigh field strengths (7T and above) compromises the reliability of quantified dynamic contrast-enhanced breast MRI. This can hamper the introduction of ultrahigh field MRI into the clinic. Compensation for this non-uniformity effect can consist of both hardware improvements and post-acquisition corrections. This paper investigated the correctable radiofrequency transmit ( ) range post-acquisition in both simulations and patient data for 7T MRI.
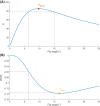
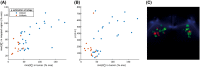
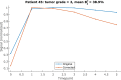
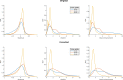
Methods: Simulations were conducted to determine the minimum level at which corrections were still beneficial because of noise amplification. Two correction strategies leading to differences in noise amplification were tested. The effect of the corrections on a 7T patient data set (N = 38) with a wide range of levels was investigated in terms of time-intensity curve types as well as washin, washout and peak enhancement values.
Results: In simulations assuming a common amount of T1 saturation, the lowest level at which the SNR of the corrected images was at least that of the original precontrast image was 43% of the nominal angle. After correction, time-intensity curve types changed in 24% of included patients, and the distribution of curve types corresponded better to the distribution found in literature. Additionally, the overlap between the distributions of washin, washout, and peak enhancement values for grade 1 and grade 2 tumors was slightly reduced.
Conclusion: Although the correctable range varies with the amount of T1 saturation, post-acquisition correction for inhomogeneous excitation was feasible down to levels of 43% of the nominal angle in vivo.
Keywords: mapping; 7T; DCE-MRI; RF field inhomogeneity; breast; flip-angle correction.
© 2019 The Authors. Magnetic Resonance in Medicine published by Wiley Periodicals, Inc. on behalf of International Society for Magnetic Resonance in Medicine.
Figures



References
-
- Kaiser WA, Zeitler E. MR Imaging of the breast ‐ fast imaging sequences with and without Gd‐Dtpa ‐ preliminary‐observations. Radiology. 1989;170:681–686. - PubMed
-
- Morris EA. Breast cancer imaging with MRI. Radiol Clin North Am. 2002;40:443–466. - PubMed
-
- Kuhl CK, Mielcareck P, Klaschik S, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999;211:101–110. - PubMed
-
- Ladd ME, Bachert P, Meyerspeer M, et al. Pros and cons of ultra‐high‐field MRI/MRS for human application. Prog Nucl Magn Reson Spectrosc. 2018;109:1–50. - PubMed
-
- Pinker K, Bogner W, Baltzer P, et al. Clinical application of bilateral high temporal and spatial resolution dynamic contrast‐enhanced magnetic resonance imaging of the breast at 7 T. Eur Radiol. 2014;24:913–920. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

