Tenuazonic acid from Stemphylium loti inhibits the plant plasma membrane H+ -ATPase by a mechanism involving the C-terminal regulatory domain
- PMID: 31880817
- PMCID: PMC7187312
- DOI: 10.1111/nph.16398
Tenuazonic acid from Stemphylium loti inhibits the plant plasma membrane H+ -ATPase by a mechanism involving the C-terminal regulatory domain
Abstract
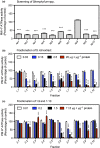
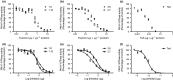
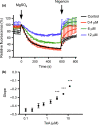
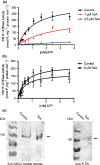
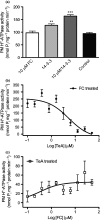
Pathogenic fungi often target the plant plasma membrane (PM) H+ -ATPase during infection. To identify pathogenic compounds targeting plant H+ -ATPases, we screened extracts from 10 Stemphylium species for their effect on H+ -ATPase activity. We identified Stemphylium loti extracts as potential H+ -ATPase inhibitors, and through chemical separation and analysis, tenuazonic acid (TeA) as a potent H+ -ATPase inhibitor. By assaying ATP hydrolysis and H+ pumping, we confirmed TeA as a H+ -ATPase inhibitor both in vitro and in vivo. To visualize in planta inhibition of the H+ -ATPase, we treated pH-sensing Arabidopsis thaliana seedlings with TeA and quantified apoplastic alkalization. TeA affected both ATPase hydrolysis and H+ pumping, supporting a direct effect on the H+ -ATPase. We demonstrated apoplastic alkalization of A. thaliana seedlings after short-term TeA treatment, indicating that TeA effectively inhibits plant PM H+ -ATPase in planta. TeA-induced inhibition was highly dependent on the regulatory C-terminal domain of the plant H+ -ATPase. Stemphylium loti is a phytopathogenic fungus. Inhibiting the plant PM H+ -ATPase results in membrane potential depolarization and eventually necrosis. The corresponding fungal H+ -ATPase, PMA1, is less affected by TeA when comparing native preparations. Fungi are thus able to target an essential plant enzyme without causing self-toxicity.
Keywords: Stemphylium loti; fusicoccin; phytotoxin; plasma membrane H+-ATPase; tenuazonic acid.
© 2019 The Authors New Phytologist © 2019 New Phytologist Trust.
Figures





References
-
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55: 373–399. - PubMed
-
- Baginski ES, Foa PP, Zak B. 1967. Determination of phosphate: Study of labile organic phosphate interference. Clinica Chimica Acta 15: 155–158.
-
- Boller T, Felix G. 2009. A renaissance of elicitors: perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annual Review of Plant Biology 60: 379–406. - PubMed
-
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Analytical Biochemistry 72: 248–254. - PubMed
-
- Chen S, Kang Y, Zhang M, Wang X, Strasser RJ, Zhou B, Qiang S. 2015. Differential sensitivity to the potential bioherbicide tenuazonic acid probed by the JIP‐test based on fast chlorophyll fluorescence kinetics. Environmental and Experimental Botany 112: 1–15.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

