Failure to confirm a sodium-glucose cotransporter 2 inhibitor-induced hematopoietic effect in non-diabetic rats with renal anemia
- PMID: 31880858
- PMCID: PMC7378420
- DOI: 10.1111/jdi.13205
Failure to confirm a sodium-glucose cotransporter 2 inhibitor-induced hematopoietic effect in non-diabetic rats with renal anemia
Abstract
Aims/introduction: Clinical studies have shown that treatment with inhibitors of sodium-glucose cotransporter 2 (SGLT2) significantly increases the hematocrit in patients with type 2 diabetes. To investigate whether SGLT2 inhibitors directly promote erythropoietin production independently on blood glucose reduction, the hematopoietic effect of the specific SGLT2 inhibitor, luseogliflozin, was examined in non-diabetic rats with renal anemia.
Materials and methods: Renal anemia was induced by treatment with adenine (200 or 600 mg/kg/day, orally for 10 days) in non-diabetic Wistar-Kyoto or Wistar rats, respectively. Luseogliflozin (10 mg/kg bodyweight) or vehicle (0.5% carboxymethyl cellulose) was then administered for 6 weeks. The hematocrit and the hemoglobin (Hb), blood urea nitrogen, plasma creatinine, and plasma erythropoietin levels were monitored.
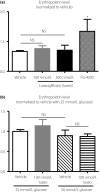
Results: Treatment with adenine decreased the hematocrit and the Hb level, which were associated with increases in the blood urea nitrogen and plasma creatinine levels. In Wistar-Kyoto rats treated with 200 mg/kg/day adenine, administration of luseogliflozin induced glycosuria, but did not change the blood urea nitrogen, plasma creatinine levels, hematocrit, Hb or plasma erythropoietin levels. Similarly, luseogliflozin treatment failed to change the hematocrit or the Hb levels in Wistar rats with renal anemia induced by 600 mg/kg/day of adenine. Plasma erythropoietin concentrations were also not different between luseogliflozin- and vehicle-treated rats. Similarly, in human erythropoietin-producing cells derived from pluripotent stem cells, luseogliflozin treatment did not change the erythropoietin level in the medium.
Conclusions: These data suggest that SGLT2 inhibitor fails to exert hematopoietic effects in non-diabetic conditions.
Keywords: Erythropoietin; Renal anemia; Sodium-glucose cotransporter 2 inhibitor.
© 2019 The Authors. Journal of Diabetes Investigation published by Asian Association for the Study of Diabetes (AASD) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
AN has received honoraria for educational meetings carried out on behalf of Taisho Co., Ltd. The other authors declare no conflict of interest.
Figures




References
-
- Gembardt F, Bartaun C, Jarzebska N, et al The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol 2014; 307: F317–325. - PubMed
-
- Wanner C, Inzucchi SE, Lachin JM, et al Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 2016; 375: 323–334. - PubMed
-
- Perkovic V, Jardine MJ, Neal B, et al Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 2019; 380: 2295–2306. - PubMed

