Cilia in cystic kidney and other diseases
- PMID: 31881326
- PMCID: PMC6953175
- DOI: 10.1016/j.cellsig.2019.109519
Cilia in cystic kidney and other diseases
Abstract
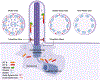
Epithelial cells lining the ducts and tubules of the kidney nephron and collecting duct have a single non-motile cilium projecting from their surface into the lumen of the tubule. These organelles were long considered vestigial remnants left as a result of evolution from a ciliated ancestor, but we now recognize them as critical sensory antennae. In the kidney, the polycystins and fibrocystin, products of the major human polycystic kidney disease genes, localize to this organelle. The polycystins and fibrocystin, through an unknown mechanism, monitor the diameter of the kidney tubules and regulate the proliferation and differentiation of the cells lining the tubule. When the polycystins, fibrocystin or cilia themselves are defective, the cell perceives this as a pro-proliferative signal, which leads to tubule dilation and cystic disease. In addition to critical roles in preventing cyst formation in the kidney, cilia are also important in cystic and fibrotic diseases of the liver and pancreas, and ciliary defects lead to a variety of developmental abnormalities that cause structural birth defects in most organs.
Keywords: Cilia; Intraflagellar transport; Kidney; Polycystic kidney disease.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors have no conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

