Ketoprofen-Based Ionic Liquids: Synthesis and Interactions with Bovine Serum Albumin
- PMID: 31881750
- PMCID: PMC6983093
- DOI: 10.3390/molecules25010090
Ketoprofen-Based Ionic Liquids: Synthesis and Interactions with Bovine Serum Albumin
Abstract
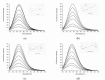
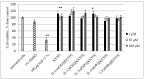
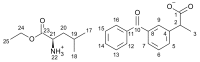
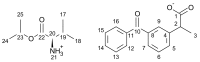
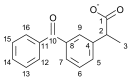
The development of ionic liquids based on active pharmaceutical ingredients (API-ILs) is a possible solution to some of the problems of solid and/or hydrophobic drugs such as low solubility and bioavailability, polymorphism and an alternative route of administration could be suggested as compared to the classical drug. Here, we report for the first time the synthesis and detailed characterization of a series of ILs containing a cation amino acid esters and anion ketoprofen (KETO-ILs). The affinity and the binding mode of the KETO-ILs to bovine serum albumin (BSA) were assessed using fluorescence spectroscopy. All compounds bind in a distance not longer than 6.14 nm to the BSA fluorophores. The estimated binding constants (KA) are in order of 105 L mol-1, which is indicative of strong drug or IL-BSA interactions. With respect to the ketoprofen-BSA system, a stronger affinity of the ILs containing l-LeuOEt, l-ValOBu, and l-ValOEt cation towards BSA is clearly seen. Fourier transformed infrared spectroscopy experiments have shown that all studied compounds induced a rearrangement of the protein molecule upon binding, which is consistent with the suggested static mechanism of BSA fluorescence quenching and formation of complexes between BSA and the drugs. All tested compounds were safe for macrophages.
Keywords: binding constants; bovine serum albumin; ionic liquids; ketoprofen; secondary structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
New insight into molecular interactions of imidazolium ionic liquids with bovine serum albumin.J Phys Chem B. 2011 Oct 27;115(42):12306-14. doi: 10.1021/jp2071925. Epub 2011 Oct 5. J Phys Chem B. 2011. PMID: 21919506
-
Spectroscopic studies on the interactions between imidazolium chloride ionic liquids and bovine serum albumin.Spectrochim Acta A Mol Biomol Spectrosc. 2013 Mar;104:377-82. doi: 10.1016/j.saa.2012.11.087. Epub 2012 Dec 5. Spectrochim Acta A Mol Biomol Spectrosc. 2013. PMID: 23274265
-
Impact of the alkyl chain length on binding of imidazolium-based ionic liquids to bovine serum albumin.Spectrochim Acta A Mol Biomol Spectrosc. 2018 May 5;196:323-333. doi: 10.1016/j.saa.2018.02.040. Epub 2018 Feb 14. Spectrochim Acta A Mol Biomol Spectrosc. 2018. PMID: 29475181
-
The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications.Int J Mol Sci. 2020 Nov 5;21(21):8298. doi: 10.3390/ijms21218298. Int J Mol Sci. 2020. PMID: 33167474 Free PMC article. Review.
-
Ionic liquids: green and tailor-made solvents in drug delivery.Drug Discov Today. 2020 May;25(5):901-908. doi: 10.1016/j.drudis.2019.09.018. Epub 2019 Oct 5. Drug Discov Today. 2020. PMID: 31593645 Review.
Cited by
-
Isopropyl Amino Acid Esters Ionic Liquids as Vehicles for Non-Steroidal Anti-Inflammatory Drugs in Potential Topical Drug Delivery Systems with Antimicrobial Activity.Int J Mol Sci. 2022 Nov 10;23(22):13863. doi: 10.3390/ijms232213863. Int J Mol Sci. 2022. PMID: 36430346 Free PMC article.
-
Salicylic Acid as Ionic Liquid Formulation May Have Enhanced Potency to Treat Some Chronic Skin Diseases.Molecules. 2021 Dec 30;27(1):216. doi: 10.3390/molecules27010216. Molecules. 2021. PMID: 35011452 Free PMC article.
-
Novel Naproxen Salts with Increased Skin Permeability.Pharmaceutics. 2021 Dec 7;13(12):2110. doi: 10.3390/pharmaceutics13122110. Pharmaceutics. 2021. PMID: 34959392 Free PMC article.
-
A Fast HPLC/UV Method for Determination of Ketoprofen in Cellular Media.ChemistryOpen. 2024 Mar;13(3):e202300147. doi: 10.1002/open.202300147. Epub 2023 Nov 13. ChemistryOpen. 2024. PMID: 37955865 Free PMC article.
-
Potential for cardiac toxicity with methylimidazolium ionic liquids.Ecotoxicol Environ Saf. 2023 Jan 1;249:114439. doi: 10.1016/j.ecoenv.2022.114439. Epub 2022 Dec 19. Ecotoxicol Environ Saf. 2023. PMID: 37272551 Free PMC article.
References
-
- Adawiyah N., Moniruzzaman M., Hawatulaila S., Goto M. Ionic liquids as a potential tool for drug delivery systems. Med. Chem. Commun. 2016;7:1881–1897. doi: 10.1039/C6MD00358C. - DOI
-
- Zhou Z., Liu C., Wan X., Fang L. Development of a w/o emulsion using ionic liquid strategy for transdermal delivery of anti-aging component α-lipoic acid: Mechanism of different ionic liquids on skin retention and efficacy evaluation. Eur. J. Pharm. Sci. 2020;141:105042. doi: 10.1016/j.ejps.2019.105042. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

