Self-care for head and neck cancer survivors with lymphedema and fibrosis: study protocol for a randomized controlled trial
- PMID: 31882012
- PMCID: PMC6935145
- DOI: 10.1186/s13063-019-3819-0
Self-care for head and neck cancer survivors with lymphedema and fibrosis: study protocol for a randomized controlled trial
Abstract
Background: Head and neck cancer (HNC) patients are at high risk for developing lymphedema and fibrosis (LEF) following cancer treatment. Once HNC patients develop LEF, they need to conduct life-long self-care to slow LEF progression and reduce associated symptom burden and functional deficits. Data demonstrate that inadequate LEF self-care may be a potentially remediable issue. The objective of this study is to explore the feasibility and preliminary efficacy of an Information-Motivation-Behavioral (IMB) Skills model-driven self-care program (SCP) to improve LEF management and reduce LEF-related symptom burden and functional impairments.
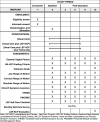
Methods/design: This is a three-arm, prospective, randomized controlled clinical trial to compare: Group 1 - Usual Care, Group 2 - Usual Care Plus LEF-SCP, and Group 3 - Usual Care Plus LEF-SCP Plus Follow-Up. Participants will be HNC survivors aged > 18 years of age, who meet predefined inclusion and exclusion criteria. A sample size of 75 participants is targeted. Interventions will be provided by trained staff. The study assessments for all groups will take place at five points: study entry then 3, 6, 9, and 12 months post enrollment. Outcome measures include: (1) feasibility (barriers to implementation, safety, and satisfaction) of the proposed intervention; (2) self-efficacy and adherence to self-care; and (3) preliminary efficacy (LEF progression, symptom burden, and functional status) of the proposed intervention.
Discussion: This will be the first study to evaluate the feasibility of a LEF-SCP in the HNC population and its impact on self-efficacy and adherence. Furthermore, it will evaluate the potential benefit of routine follow-up on adherence and fidelity to the self-care protocol. We expect that the trial will provide evidence supporting the feasibility of a LEF self-care program. In addition, we anticipate that preliminary data will support improved outcomes including increased adherence and fidelity, and decreased LEF-associated symptoms.
Trial registration: ClinicalTrials.gov, a service of the US National Institute of Health (NCT03030859). Registered on 22 January 2017.
Keywords: Fibrosis; Head and neck cancer; Lymphedema; Self-care; Self-management; Survivorship.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- American Cancer Society. Cancer facts and figures 2010. Atlanta, GA: American Cancer Society; 2010. Accessed: 1 Mar 2016. Available from: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/docume...
-
- American Cancer Society. Cancer facts and figures 2019. Atlanta, GA: American Cancer Society; 2019. Accessed: 1 Mar 2019. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-...
-
- Haddad RI, Limaye S. Overview of approach to long-term survivors of head and neck cancer. In: Post TW, (Ed), UpToDate. Accessed 1 Mar 2016. Waltham, MA: UpToDate; 2016.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical