Pulmonary surfactant lipids inhibit infections with the pandemic H1N1 influenza virus in several animal models
- PMID: 31882535
- PMCID: PMC7008372
- DOI: 10.1074/jbc.RA119.012053
Pulmonary surfactant lipids inhibit infections with the pandemic H1N1 influenza virus in several animal models
Abstract
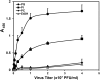
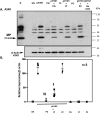
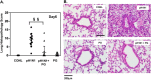
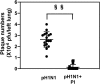
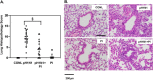
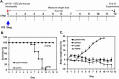
The influenza A (H1N1)pdm09 outbreak in 2009 exemplified the problems accompanying the emergence of novel influenza A virus (IAV) strains and their unanticipated virulence in populations with no pre-existing immunity. Neuraminidase inhibitors (NAIs) are currently the drugs of choice for intervention against IAV outbreaks, but there are concerns that NAI-resistant viruses can transmit to high-risk populations. These issues highlight the need for new approaches that address the annual influenza burden. In this study, we examined whether palmitoyl-oleoyl-phosphatidylglycerol (POPG) and phosphatidylinositol (PI) effectively antagonize (H1N1)pdm09 infection. POPG and PI markedly suppressed cytopathic effects and attenuated viral gene expression in (H1N1)pdm09-infected Madin-Darby canine kidney cells. POPG and PI bound to (H1N1)pdm09 with high affinity and disrupted viral spread from infected to noninfected cells in tissue culture and also reduced (H1N1)pdm09 propagation by a factor of 102 after viral infection was established in vitro In a mouse infection model of (H1N1)pdm09, POPG and PI significantly reduced lung inflammation and viral burden. Of note, when mice were challenged with a typically lethal dose of 1000 plaque-forming units of (H1N1)pdm09, survival after 10 days was 100% (14 of 14 mice) with the POPG treatment compared with 0% (0 of 14 mice) without this treatment. POPG also significantly reduced inflammatory infiltrates and the viral burden induced by (H1N1)pdm09 infection in a ferret model. These findings indicate that anionic phospholipids potently and efficiently disrupt influenza infections in animal models.
Keywords: antiviral agent; host defense; inflammation; influenza; innate immunity; phosphatidylglycerol; phosphatidylinositol; phospholipids; pulmonary surfactant; treatments; virology.
© 2020 Numata et al.
Conflict of interest statement
M. N. and D. R. V. are the co-inventors of the patent
Figures








References
-
- Shinde V., Bridges C. B., Uyeki T. M., Shu B., Balish A., Xu X., Lindstrom S., Gubareva L. V., Deyde V., Garten R. J., Harris M., Gerber S., Vagasky S., Smith F., Pascoe N., et al. (2009) Triple-reassortant swine influenza A (H1) in humans in the United States, 2005–2009. N. Engl. J. Med. 360, 2616–2625 10.1056/NEJMoa0903812 - DOI - PubMed
-
- Garten R. J., Davis C. T., Russell C. A., Shu B., Lindstrom S., Balish A., Sessions W. M., Xu X., Skepner E., Deyde V., Okomo-Adhiambo M., Gubareva L., Barnes J., Smith C. B., Emery S. L., et al. (2009) Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325, 197–201 10.1126/science.1176225 - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention (CDC). (2012) Update: Influenza activity–United States, October 2, 2011–February 11, 2012. MMWR Morb. Mortal. Wkly. Rep. 61, 123–128 - PubMed
-
- Epperson S., Blanton L., Kniss K., Mustaquim D., Steffens C., Wallis T., Dhara R., Leon M., Perez A., Chaves S. S., Elal A. A., Gubareva L., Xu X., Villanueva J., Bresee J., et al. (2014) Influenza activity–United States, 2013–14 season and composition of the 2014–15 influenza vaccines. MMWR Morb. Mortal. Wkly. Rep. 63, 483–490 - PMC - PubMed
-
- Blanton L., Dugan V. G., Abd Elal A. I., Alabi N., Barnes J., Brammer L., Budd A. P., Burns E., Cummings C. N., Garg S., Garten R., Gubareva L., Kniss K., Kramer N., O'Halloran A., et al. (2019) Update: Influenza activity–United States, September 30, 2018-February 2, 2019. MMWR Morb. Mortal. Wkly. Rep. 68, 125–134 10.15585/mmwr.mm6806a1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

