Crystal structure of the N-terminal domain of the trypanosome flagellar protein BILBO1 reveals a ubiquitin fold with a long structured loop for protein binding
- PMID: 31882537
- PMCID: PMC7008359
- DOI: 10.1074/jbc.RA119.010768
Crystal structure of the N-terminal domain of the trypanosome flagellar protein BILBO1 reveals a ubiquitin fold with a long structured loop for protein binding
Abstract
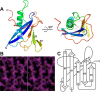
Trypanosoma brucei is a protist parasite causing sleeping sickness and nagana in sub-Saharan Africa. T. brucei has a single flagellum whose base contains a bulblike invagination of the plasma membrane called the flagellar pocket (FP). Around the neck of the FP on its cytoplasmic face is a structure called the flagellar pocket collar (FPC), which is essential for FP biogenesis. BILBO1 was the first characterized component of the FPC in trypanosomes. BILBO1's N-terminal domain (NTD) plays an essential role in T. brucei FPC biogenesis and is thus vital for the parasite's survival. Here, we report a 1.6-Å resolution crystal structure of TbBILBO1-NTD, which revealed a conserved horseshoe-like hydrophobic pocket formed by an unusually long loop. Results from mutagenesis experiments suggested that another FPC protein, FPC4, interacts with TbBILBO1 by mainly contacting its three conserved aromatic residues Trp-71, Tyr-87, and Phe-89 at the center of this pocket. Our findings disclose the binding site of TbFPC4 on TbBILBO1-NTD, which may provide a basis for rational drug design targeting BILBO1 to combat T. brucei infections.
Keywords: BILBO1; FPC4; Trypanosoma brucei; crystal structure; crystallography; cytoskeleton; flagellar pocket collar; parasite; protein structure; protein-protein interaction; structural biology; ubiquitin; ubiquitin fold.
© 2020 Vidilaseris et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

