Structural and mechanistic insights into Hsp104 function revealed by synchrotron X-ray footprinting
- PMID: 31882541
- PMCID: PMC7008382
- DOI: 10.1074/jbc.RA119.011577
Structural and mechanistic insights into Hsp104 function revealed by synchrotron X-ray footprinting
Abstract
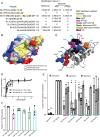
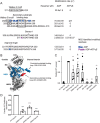
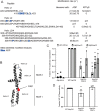
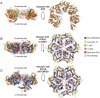
Hsp104 is a hexameric AAA+ ring translocase, which drives protein disaggregation in nonmetazoan eukaryotes. Cryo-EM structures of Hsp104 have suggested potential mechanisms of substrate translocation, but precisely how Hsp104 hexamers disaggregate proteins remains incompletely understood. Here, we employed synchrotron X-ray footprinting to probe the solution-state structures of Hsp104 monomers in the absence of nucleotide and Hsp104 hexamers in the presence of ADP or ATPγS (adenosine 5'-O-(thiotriphosphate)). Comparing side-chain solvent accessibilities between these three states illuminated aspects of Hsp104 structure and guided design of Hsp104 variants to probe the disaggregase mechanism in vitro and in vivo We established that Hsp104 hexamers switch from a more-solvated state in ADP to a less-solvated state in ATPγS, consistent with switching from an open spiral to a closed ring visualized by cryo-EM. We pinpointed critical N-terminal domain (NTD), NTD-nucleotide-binding domain 1 (NBD1) linker, NBD1, and middle domain (MD) residues that enable intrinsic disaggregase activity and Hsp70 collaboration. We uncovered NTD residues in the loop between helices A1 and A2 that can be substituted to enhance disaggregase activity. We elucidated a novel potentiated Hsp104 MD variant, Hsp104-RYD, which suppresses α-synuclein, fused in sarcoma (FUS), and TDP-43 toxicity. We disambiguated a secondary pore-loop in NBD1, which collaborates with the NTD and NBD1 tyrosine-bearing pore-loop to drive protein disaggregation. Finally, we defined Leu-601 in NBD2 as crucial for Hsp104 hexamerization. Collectively, our findings unveil new facets of Hsp104 structure and mechanism. They also connect regions undergoing large changes in solvation to functionality, which could have profound implications for protein engineering.
Keywords: ATPases associated with diverse cellular activities (AAA); FUS; Hsp104; TDP-43; disaggregase; heat-shock protein (HSP); mass spectrometry (MS); mutagenesis; protein chemical modification; α-synuclein.
© 2020 Sweeny et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures





References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

