The Local Paracrine Actions of the Pancreatic α-Cell
- PMID: 31882565
- PMCID: PMC7085245
- DOI: 10.2337/dbi19-0002
The Local Paracrine Actions of the Pancreatic α-Cell
Abstract
Secretion of glucagon from the pancreatic α-cells is conventionally seen as the first and most important defense against hypoglycemia. Recent findings, however, show that α-cell signals stimulate insulin secretion from the neighboring β-cell. This article focuses on these seemingly counterintuitive local actions of α-cells and describes how they impact islet biology and glucose metabolism. It is mostly based on studies published in the last decade on the physiology of α-cells in human islets and incorporates results from rodents where appropriate. As this and the accompanying articles show, the emerging picture of α-cell function is one of increased complexity that needs to be considered when developing new therapies aimed at promoting islet function in the context of diabetes.
© 2019 by the American Diabetes Association.
Figures
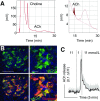
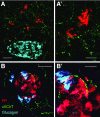
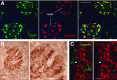

References
-
- Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes 1988;37:413–420 - PubMed
-
- Brissova M, Fowler MJ, Nicholson WE, et al. . Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 2005;53:1087–1097 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

