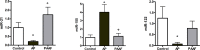Acute pancreatitis promotes the generation of two different exosome populations
- PMID: 31882721
- PMCID: PMC6934470
- DOI: 10.1038/s41598-019-56220-5
Acute pancreatitis promotes the generation of two different exosome populations
Abstract
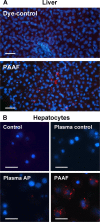
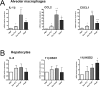
Exosomes are small extracellular vesicles that act as intercellular messengers. Previous studies revealed that, during acute pancreatitis, circulating exosomes could reach the alveolar compartment and activate macrophages. However, proteomic analysis suggested that the most likely origin of these exosomes could be the liver instead of the pancreas. The present study aimed to characterize the exosomes released by pancreas to pancreatitis-associated ascitic fluid (PAAF) as well as those circulating in plasma in an experimental model of taurocholate-induced acute pancreatitis in rats. We provide evidence that during acute pancreatitis two different populations of exosomes are generated with relevant differences in cell distribution, protein and microRNA content as well as different implications in their physiological effects. During pancreatitis plasma exosomes, but not PAAF exosomes, are enriched in the inflammatory miR-155 and show low levels of miR-21 and miR-122. Mass spectrometry-based proteomic analysis showed that PAAF exosomes contains 10-30 fold higher loading of histones and ribosomal proteins compared to plasma exosomes. Finally, plasma exosomes have higher pro-inflammatory activity on macrophages than PAAF exosomes. These results confirm the generation of two different populations of exosomes during acute pancreatitis. Deep understanding of their specific functions will be necessary to use them as therapeutic targets at different stages of the disease.
Conflict of interest statement
The authors declare no competing interests.
Figures