In Vivo Activity of Amodiaquine against Ebola Virus Infection
- PMID: 31882748
- PMCID: PMC6934550
- DOI: 10.1038/s41598-019-56481-0
In Vivo Activity of Amodiaquine against Ebola Virus Infection
Abstract
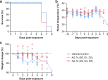
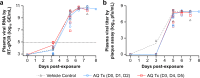
During the Ebola virus disease (EVD) epidemic in Western Africa (2013‒2016), antimalarial treatment was administered to EVD patients due to the high coexisting malaria burden in accordance with World Health Organization guidelines. In an Ebola treatment center in Liberia, EVD patients receiving the combination antimalarial artesunate-amodiaquine had a lower risk of death compared to those treated with artemether-lumefantrine. As artemether and artesunate are derivatives of artemisinin, the beneficial anti-Ebola virus (EBOV) effect observed could possibly be attributed to the change from lumefantrine to amodiaquine. Amodiaquine is a widely used antimalarial in the countries that experience outbreaks of EVD and, therefore, holds promise as an approved drug that could be repurposed for treating EBOV infections. We investigated the potential anti-EBOV effect of amodiaquine in a well-characterized nonhuman primate model of EVD. Using a similar 3-day antimalarial dosing strategy as for human patients, plasma concentrations of amodiaquine in healthy animals were similar to those found in humans. However, the treatment regimen did not result in a survival benefit or decrease of disease signs in EBOV-infected animals. While amodiaquine on its own failed to demonstrate efficacy, we cannot exclude potential therapeutic value of amodiaquine when used in combination with artesunate or another antiviral.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- World Health Organization. Clinical management of patients with viral haemorrhagic fever, https://www.who.int/csr/resources/publications/clinical-management-patie... (2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

