c-Cbl targets PD-1 in immune cells for proteasomal degradation and modulates colorectal tumor growth
- PMID: 31882749
- PMCID: PMC6934810
- DOI: 10.1038/s41598-019-56208-1
c-Cbl targets PD-1 in immune cells for proteasomal degradation and modulates colorectal tumor growth
Abstract
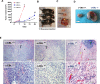
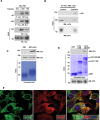
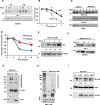
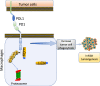
Casitas B lymphoma (c-Cbl) is an E3 ubiquitin ligase and a negative regulator of colorectal cancer (CRC). Despite its high expression in immune cells, the effect of c-Cbl on the tumor microenvironment remains poorly understood. Here we demonstrate that c-Cbl alters the tumor microenvironment and suppresses Programmed cell death-1 (PD-1) protein, an immune checkpoint receptor. Using syngeneic CRC xenografts, we observed significantly higher growth of xenografts and infiltrating immune cells in c-Cbl+/- compared to c-Cbl+/+ mice. Tumor-associated CD8+ T-lymphocytes and macrophages of c-Cbl+/- mice showed 2-3-fold higher levels of PD-1. Functionally, macrophages from c-Cbl+/- mice showed a 4-5-fold reduction in tumor phagocytosis, which was restored with an anti-PD-1 neutralizing antibody suggesting regulation of PD-1 by c-Cbl. Further mechanistic probing revealed that C-terminus of c-Cbl interacted with the cytoplasmic tail of PD-1. c-Cbl destabilized PD-1 through ubiquitination- proteasomal degradation depending on c-Cbl's RING finger function. This data demonstrates c-Cbl as an E3 ligase of PD-1 and a regulator of tumor microenvironment, both of which were unrecognized components of its tumor suppressive activity. Advancing immune checkpoint and c-Cbl biology, our study prompts for probing of PD-1 regulation by c-Cbl in conditions driven by immune checkpoint abnormalities such as cancers and autoimmune diseases.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
- T32 HL007224-40/U.S. Department of Health & Human Services | NIH | National Heart, Lung, and Blood Institute (NHLBI)/International
- R21 CA193958/CA/NCI NIH HHS/United States
- R01 HL132325/HL/NHLBI NIH HHS/United States
- R21 CA191970/CA/NCI NIH HHS/United States
- R01CA175382/Division of Cancer Prevention, National Cancer Institute (NCI Division of Cancer Prevention)/International
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

