Biological Carbon Recovery from Sugar Refinery Washing Water into Microalgal DHA: Medium Optimization and Stress Induction
- PMID: 31882916
- PMCID: PMC6934592
- DOI: 10.1038/s41598-019-56406-x
Biological Carbon Recovery from Sugar Refinery Washing Water into Microalgal DHA: Medium Optimization and Stress Induction
Abstract
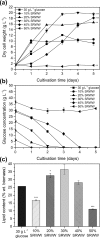
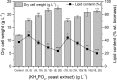
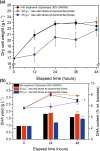
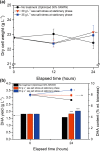
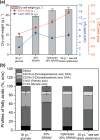
Sugar refinery washing water (SRWW) contains abundant levels of carbon sources and lower levels of contaminants than other types of wastewater, which makes it ideal for heterotrophic cultivation of microalgae. Here, carbon sources in SRWW were utilized for conversion into the form of value-added docosahexaenoic acid (DHA) using Aurantiochytrium sp. KRS101. Since SRWW is not a defined medium, serial optimizations were performed to maximize the biomass, lipid, and DHA yields by adjusting the nutrient (carbon, nitrogen, and phosphorus) concentrations as well as the application of salt stress. Optimum growth performance was achieved with 30% dilution of SRWW containing a total organic carbon of 95,488 mg L-1. Increasing the nutrient level in the medium by supplementation of 9 g L-1 KH2PO4 and 20 g L-1 yeast extract further improved the biomass yield by an additional 14%, albeit at the expense of a decrease in the lipid content. Maximum biomass, lipid, and DHA yields (22.9, 6.33, and 2.03 g L-1, respectively) were achieved when 35 g L-1 sea salt was applied on a stationary phase for osmotic stress. These results demonstrate the potential of carbon-rich sugar refinery washing water for DHA production using Aurantiochytrium sp. KRS101 and proper cultivation strategy.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Production of lipids containing high levels of docosahexaenoic acid by a newly isolated microalga, Aurantiochytrium sp. KRS101.Appl Biochem Biotechnol. 2011 Aug;164(8):1468-80. doi: 10.1007/s12010-011-9227-x. Epub 2011 Mar 22. Appl Biochem Biotechnol. 2011. PMID: 21424706
-
Response surface optimization of culture medium for enhanced docosahexaenoic acid production by a Malaysian thraustochytrid.Sci Rep. 2015 Feb 27;5:8611. doi: 10.1038/srep08611. Sci Rep. 2015. PMID: 25721623 Free PMC article.
-
A novel fed-batch process based on the biology of Aurantiochytrium sp. KRS101 for the production of biodiesel and docosahexaenoic acid.Bioresour Technol. 2013 May;135:269-74. doi: 10.1016/j.biortech.2012.10.139. Epub 2012 Nov 5. Bioresour Technol. 2013. PMID: 23206808
-
Production of docosahexaenoic acid by a novel isolated Aurantiochytrium sp. 6-2 using fermented defatted soybean as a nitrogen source for sustainable fish feed development.Biosci Biotechnol Biochem. 2024 May 22;88(6):696-704. doi: 10.1093/bbb/zbae035. Biosci Biotechnol Biochem. 2024. PMID: 38520162
-
Microalgae systems - environmental agents for wastewater treatment and further potential biomass valorisation.J Environ Manage. 2023 Jul 1;337:117678. doi: 10.1016/j.jenvman.2023.117678. Epub 2023 Mar 21. J Environ Manage. 2023. PMID: 36948147 Review.
Cited by
-
Straw mulch improves soil carbon and nitrogen cycle by mediating microbial community structure and function in the maize field.Front Microbiol. 2023 Jul 18;14:1217966. doi: 10.3389/fmicb.2023.1217966. eCollection 2023. Front Microbiol. 2023. PMID: 37533822 Free PMC article.
-
Last Decade Insights in Exploiting Marine Microorganisms as Sources of New Bioactive Natural Products.Mar Drugs. 2025 Mar 7;23(3):116. doi: 10.3390/md23030116. Mar Drugs. 2025. PMID: 40137302 Free PMC article. Review.
-
Advancing sustainable agriculture: the potential of seaweed-derived bio pesticides from marine biomass.Bioresour Bioprocess. 2025 Mar 22;12(1):22. doi: 10.1186/s40643-025-00849-w. Bioresour Bioprocess. 2025. PMID: 40120004 Free PMC article. Review.
References
-
- Borowitzka MA. High-value products from microalgae—their development and commercialisation. J. Appl. Phycol. 2013;25:743–756. doi: 10.1007/s10811-013-9983-9. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

