A microfluidics-based wound-healing assay for studying the effects of shear stresses, wound widths, and chemicals on the wound-healing process
- PMID: 31882962
- PMCID: PMC6934480
- DOI: 10.1038/s41598-019-56753-9
A microfluidics-based wound-healing assay for studying the effects of shear stresses, wound widths, and chemicals on the wound-healing process
Abstract
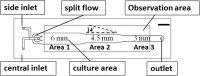
Collective cell migration plays important roles in various physiological processes. To investigate this collective cellular movement, various wound-healing assays have been developed. In these assays, a "wound" is created mechanically, chemically, optically, or electrically out of a cellular monolayer. Most of these assays are subject to drawbacks of run-to-run variations in wound size/shape and damages to cells/substrate. Moreover, in all these assays, cells are cultured in open, static (non-circulating) environments. In this study, we reported a microfluidics-based wound-healing assay by using the trypsin flow-focusing technique. Fibroblasts were first cultured inside this chip to a cellular monolayer. Then three parallel fluidic flows (containing normal medium and trypsin solution) were introduced into the channels, and cells exposed to protease trypsin were enzymatically detached from the surface. Wounds of three different widths were generated, and subsequent wound-healing processes were observed. This assay is capable of creating three or more wounds of different widths for investigating the effects of various physical and chemical stimuli on wound-healing speeds. The effects of shear stresses, wound widths, and β-lapachone (a wound healing-promoting chemical) on wound-healing speeds were studied. It was found that the wound-healing speed (total area healed per unit time) increased with increasing shear stress and wound width, but under a shear stress of 0.174 mPa the linear healing speed (percent area healed per unit time) was independent of the wound width. Also, the addition of β-lapachone up to 0.5 μM did not accelerate wound healing. This microfluidics-based assay can definitely help in understanding the mechanisms of the wound-healing process and developing new wound-healing therapies.
Conflict of interest statement
The authors declare no competing interests.
Figures







Similar articles
-
A Wound-Healing Assay Based on Ultraviolet Light Ablation.SLAS Technol. 2017 Feb;22(1):36-43. doi: 10.1177/2211068216646741. Epub 2016 Jul 10. SLAS Technol. 2017. PMID: 27139694
-
A passive pump-assisted microfluidic assay for quantifying endothelial wound healing in response to fluid shear stress.Electrophoresis. 2022 Nov;43(21-22):2195-2205. doi: 10.1002/elps.202200104. Epub 2022 Aug 9. Electrophoresis. 2022. PMID: 35899363
-
Fibroblast Derived Skin Wound Healing Modeling on Chip under the Influence of Micro-Capillary Shear Stress.Micromachines (Basel). 2022 Feb 16;13(2):305. doi: 10.3390/mi13020305. Micromachines (Basel). 2022. PMID: 35208429 Free PMC article.
-
Microfluidic and Lab-on-a-Chip Systems for Cutaneous Wound Healing Studies.Pharmaceutics. 2021 May 26;13(6):793. doi: 10.3390/pharmaceutics13060793. Pharmaceutics. 2021. PMID: 34073346 Free PMC article. Review.
-
Advances in wound-healing assays for probing collective cell migration.J Lab Autom. 2012 Feb;17(1):59-65. doi: 10.1177/2211068211426550. J Lab Autom. 2012. PMID: 22357609 Review.
Cited by
-
The Effects of Combined Exposure to Simulated Microgravity, Ionizing Radiation, and Cortisol on the In Vitro Wound Healing Process.Cells. 2023 Jan 7;12(2):246. doi: 10.3390/cells12020246. Cells. 2023. PMID: 36672184 Free PMC article.
-
Response of cells and tissues to shear stress.J Cell Sci. 2023 Sep 15;136(18):jcs260985. doi: 10.1242/jcs.260985. Epub 2023 Sep 25. J Cell Sci. 2023. PMID: 37747423 Free PMC article. Review.
-
Development of Skin-On-A-Chip Platforms for Different Utilizations: Factors to Be Considered.Micromachines (Basel). 2021 Mar 10;12(3):294. doi: 10.3390/mi12030294. Micromachines (Basel). 2021. PMID: 33802208 Free PMC article. Review.
-
A microfluidic chip carrier including temperature control and perfusion system for long-term cell imaging.HardwareX. 2021 Nov 6;10:e00245. doi: 10.1016/j.ohx.2021.e00245. eCollection 2021 Oct. HardwareX. 2021. PMID: 35607686 Free PMC article.
-
A Microfluidic System for Real-Time Monitoring and In Situ Metabolite Detection of Plasma-Enhanced Wound Healing.Biomolecules. 2025 Jul 25;15(8):1077. doi: 10.3390/biom15081077. Biomolecules. 2025. PMID: 40867522 Free PMC article.
References
-
- Chen HC. Boyden chamber assay. Methods Mol Biol. 2005;294:15–22. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

