High degree of polyclonality hinders somatic mutation calling in lung brush samples of COPD cases and controls
- PMID: 31882973
- PMCID: PMC6934450
- DOI: 10.1038/s41598-019-56618-1
High degree of polyclonality hinders somatic mutation calling in lung brush samples of COPD cases and controls
Abstract
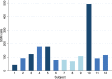
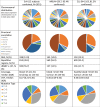
Chronic obstructive pulmonary disease (COPD) is induced by cigarette smoking and characterized by inflammation of airway tissue. Since smokers with COPD have a higher risk of developing lung cancer than those without, we hypothesized that they carry more mutations in affected tissue. We called somatic mutations in airway brush samples from medium-coverage whole genome sequencing data from healthy never and ex-smokers (n = 8), as well as from ex-smokers with variable degrees of COPD (n = 4). Owing to the limited concordance of resulting calls between the applied tools we built a consensus, a strategy that was validated with high accuracy for cancer data. However, consensus calls showed little promise of representing true positives due to low mappability of corresponding sequence reads and high overlap with positions harbouring known genetic polymorphisms. A targeted re-sequencing approach suggested that only few mutations would survive stringent verification testing and that our data did not allow the inference of any difference in the mutational load of bronchial brush samples between former smoking COPD cases and controls. High polyclonality in airway brush samples renders medium-depth sequencing insufficient to provide the resolution to detect somatic mutations. Deep sequencing data of airway biopsies are needed to tackle the question.
Conflict of interest statement
T.G. reports personal fees from Astra Zeneca, Berlin-Chemie, Boehringer-Ingelheim, Chiesi, CSL-Behring, GSK, Novartis, and grants and personal fees from Grifols, outside the submitted work; and reports grants from the European Union during the conduct of the study. The other authors declare no competing financial and/or non-financial interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

