Age and DNA methylation subgroup as potential independent risk factors for treatment stratification in children with atypical teratoid/rhabdoid tumors
- PMID: 31883020
- PMCID: PMC7339901
- DOI: 10.1093/neuonc/noz244
Age and DNA methylation subgroup as potential independent risk factors for treatment stratification in children with atypical teratoid/rhabdoid tumors
Abstract
Background: Controversy exists as to what may be defined as standard of care (including markers for stratification) for patients with atypical teratoid/rhabdoid tumors (ATRTs). The European Rhabdoid Registry (EU-RHAB) recruits uniformly treated patients and offers standardized genetic and DNA methylation analyses.
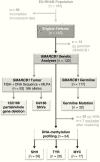
Methods: Clinical, genetic, and treatment data of 143 patients from 13 European countries were analyzed (2009-2017). Therapy consisted of surgery, anthracycline-based induction, and either radiotherapy or high dose chemotherapy following a consensus among European experts. Fluorescence in situ hybridization, multiplex ligation-dependent probe amplification, and sequencing were employed for assessment of somatic and germline mutations in SWItch/sucrose nonfermentable related, matrix associated, actin dependent regulator of chromatin, subfamily B (SMARCB1). Molecular subgroups (ATRT-SHH, ATRT-TYR, and ATRT-MYC) were determined using DNA methylation arrays, resulting in profiles of 84 tumors.
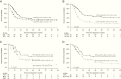
Results: Median age at diagnosis of 67 girls and 76 boys was 29.5 months. Five-year overall survival (OS) and event-free survival (EFS) were 34.7 ± 4.5% and 30.5 ± 4.2%, respectively. Tumors displayed allelic partial/whole gene deletions (66%; 122/186 alleles) or single nucleotide variants (34%; 64/186 alleles) of SMARCB1. Germline mutations were detected in 26% of ATRTs (30/117). The patient cohort consisted of 47% ATRT-SHH (39/84), 33% ATRT-TYR (28/84), and 20% ATRT-MYC (17/84). Age <1 year, non-TYR signature (ATRT-SHH or -MYC), metastatic or synchronous tumors, germline mutation, incomplete remission, and omission of radiotherapy were negative prognostic factors in univariate analyses (P < 0.05). An adjusted multivariate model identified age <1 year and a non-TYR signature as independent negative predictors of OS: high risk (<1 y + non-TYR; 5-y OS = 0%), intermediate risk (<1 y + ATRT-TYR or ≥1 y + non-TYR; 5-y OS = 32.5 ± 8.7%), and standard risk (≥1 y + ATRT-TYR, 5-y OS = 71.5 ± 12.2%).
Conclusions: Age and molecular subgroup status are independent risk factors for survival in children with ATRT. Our model warrants validation within future clinical trials.
Keywords: ATRT; DNA methylation profiling; European Rhabdoid Tumor Registry; SMARCB1; prognosis.
© The Author(s) 2019. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures


References
-
- Hasselblatt M, Isken S, Linge A, et al. . High-resolution genomic analysis suggests the absence of recurrent genomic alterations other than SMARCB1 aberrations in atypical teratoid/rhabdoid tumors. Genes Chromosomes Cancer. 2013;52(2):185–190. - PubMed
-
- Hasselblatt M, Nagel I, Oyen F, et al. . SMARCA4-mutated atypical teratoid/rhabdoid tumors are associated with inherited germline alterations and poor prognosis. Acta Neuropathol. 2014;128(3): 453–456. - PubMed

