T-cell exhaustion in HIV infection
- PMID: 31883174
- PMCID: PMC7003858
- DOI: 10.1111/imr.12823
T-cell exhaustion in HIV infection
Abstract
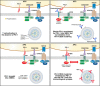
The T-cell response is central in the adaptive immune-mediated elimination of pathogen-infected and/or cancer cells. This activated T-cell response can inflict an overwhelming degree of damage to the targeted cells, which in most instances leads to the control and elimination of foreign invaders. However, in conditions of chronic infection, persistent exposure of T cells to high levels of antigen results in a severe T-cell dysfunctional state called exhaustion. T-cell exhaustion leads to a suboptimal immune-mediated control of multiple viral infections including the human immunodeficiency virus (HIV). In this review, we will discuss the role of T-cell exhaustion in HIV disease progression, the long-term defect of T-cell function even in aviremic patients on antiretroviral therapy (ART), the role of exhaustion-specific markers in maintaining a reservoir of latently infected cells, and exploiting these markers in HIV cure strategies.
Keywords: HIV; PD-1; T-cell exhaustion; chronic viral infection; immune checkpoint inhibitors.
© 2019 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare no conflict of interest with this review.
Figures


References
-
- Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305‐334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

